|
Page History
How to Record New Site Subject Accrual Counts
| Info | ||
|---|---|---|
| title | Note | : The default accrual type for Complete Non-Interventional studies is Subject. However, if no accruals have been reported for this study, the default accrual reporting type can be changed to Summary accrual type. If accrual has been reported, then the following actions are required:
|
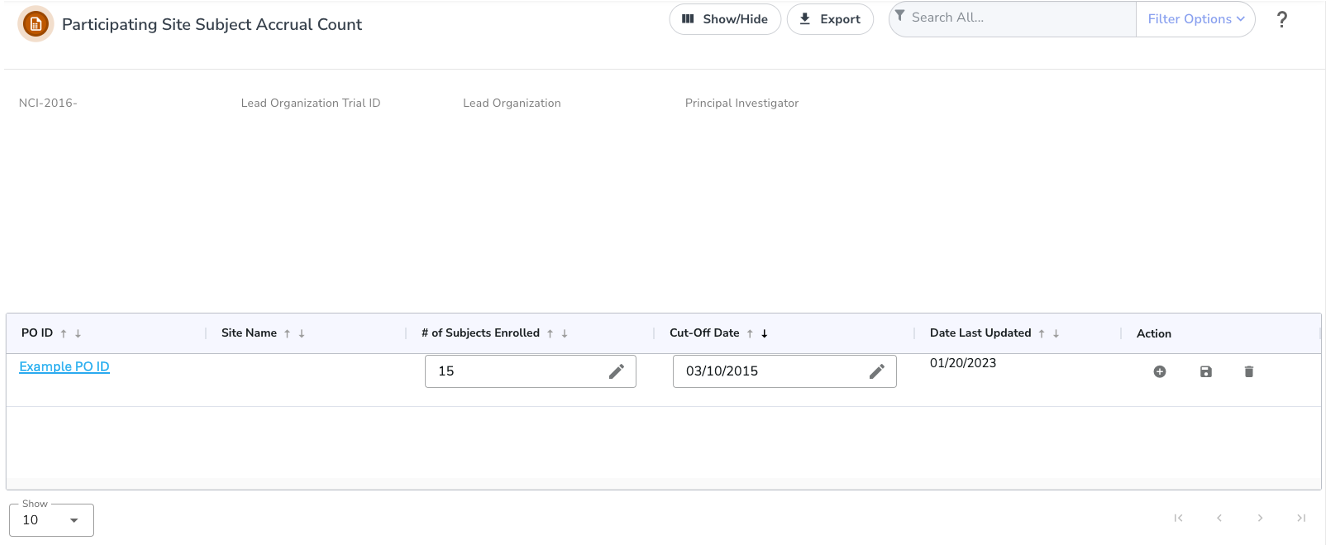
Select the trial you want to work with by following instructions in Searching for and Selecting Your Trials, and clicking the corresponding NCI Trial Identifier link. Ensure that the trial selected is a Summary accrual type. The Participating Site Subject Accrual Count page appears.
HTML Comment hidden true Screenshot TBD.
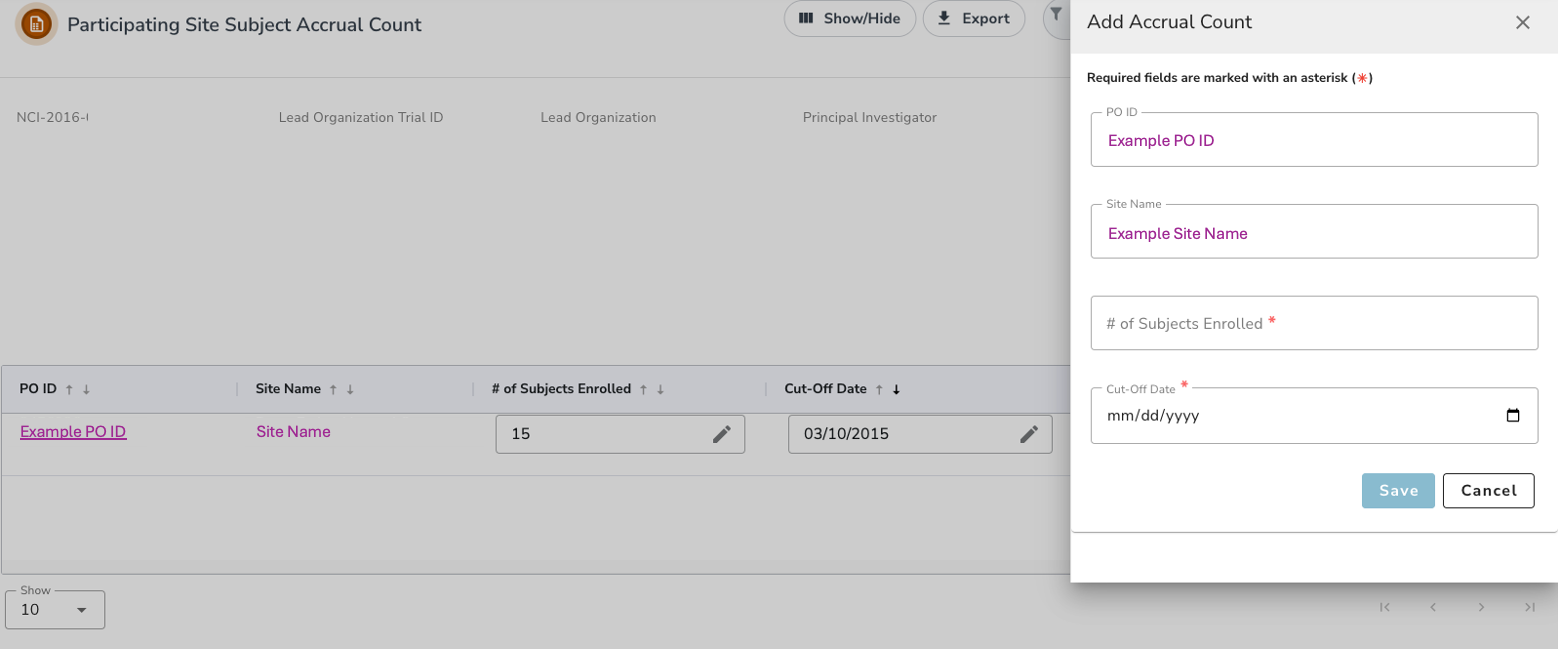
- To record the accrual information for the first time, in the Actions column, click on the Plus “+” icon . and the Add Accrual Count panel should appear.
- In the # of Subjects Enrolled field, enter the number of subjects currently enrolled in studies at your site.
- In the Cut-Off Date field, specify the date from which the cumulative accrual count was calculated. If you do not specify a cut-off date, the system will not allow you to save.
- Click Save. A message "Accrual Count has been added" will appear indicating that the record has been updated successfully.
- Designated Cancer Center users may view, add, edit or delete an entry. Clicking on the PO ID will allow the user to expand the most recent summary record and view the history of updates made to the accrual count/cutoff history records.
...