This section introduces you to NCI Clinical Trials Reporting Program (CTRP) Registration, and provides instructions for registering for an account.
About Registration
Registration provides cancer clinical trial access to researchers who have CTRP accounts. It enables these users to register, amend, and update trials one at a time, and to view details of existing trials submitted by members of the cancer research community. You can register Complete and Abbreviated interventional and non-interventional trials.
About Clinical Trial Details
Registration captures clinical trial details, a sanctioned set of key data elements. This set of recorded data enables the research community to share and analyze standardized clinical trial details.
The glossary on the CTRP web site at http://www.cancer.gov/aboutnci/organization/ccct/ctrp/glossary provides definitions, attributes, and examples of metadata associated with trials. You can access other CTRP resources from the same page.
As a CTRP account holder, you can search for and review a subset of registered data that has been submitted to CTRP and validated by the Clinical Trials Reporting Office (CTRO).
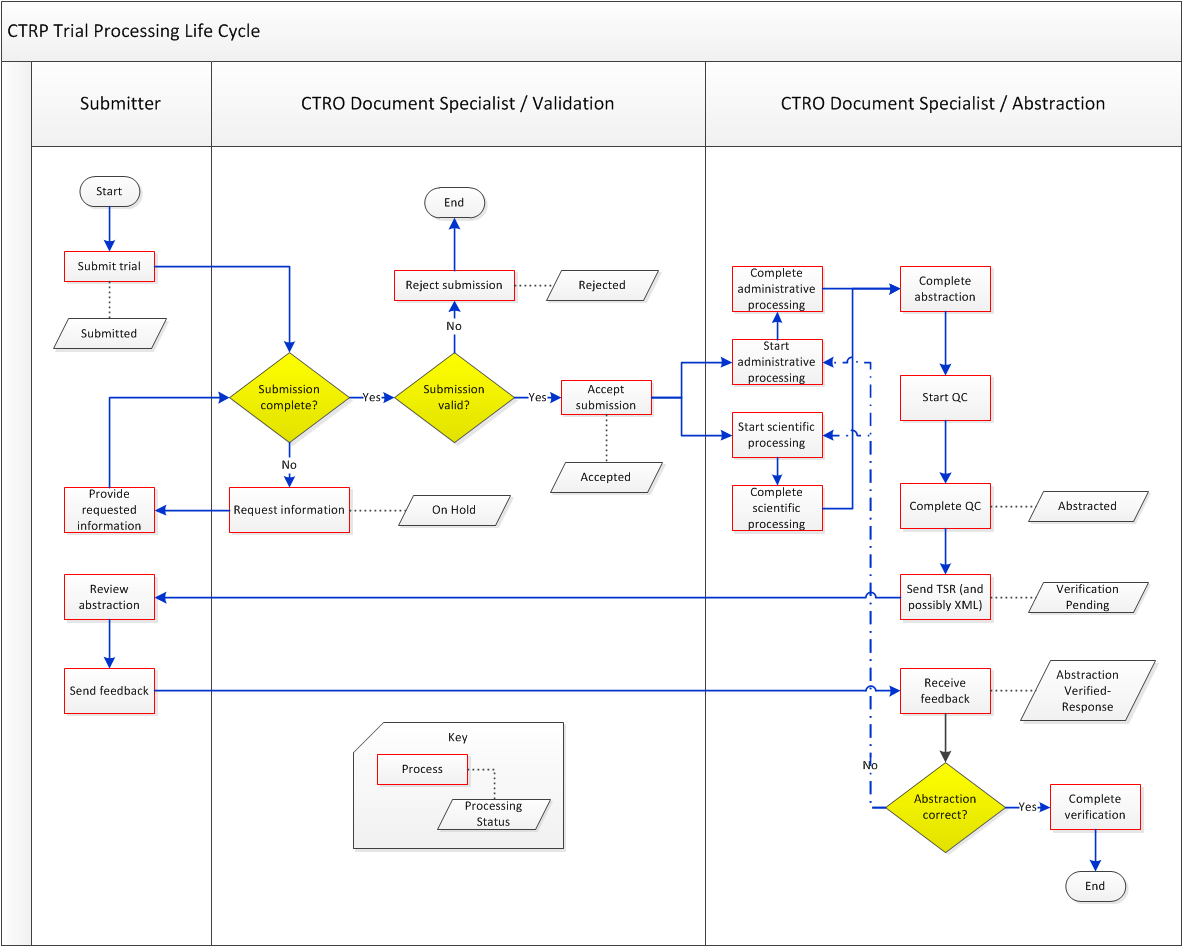
Typical Life Cycle of a Trial
This section describes, in words and visually, the typical life cycle of a trial:
- The submitter submits the trial. The trial has a processing status of Submitted.
- The CTRO Document Specialist performs validation on the Submitted trial:
- If the trial is not complete, the specialist sends a request to the submitter for information. The trial has a processing status of On Hold. When the CTRO receives the requested information, a Document Specialist takes the trial off hold. The trial's processing status returns to the previous status, which in this case would be Submitted.
- If the trial is not valid, the specialist rejects the submission. The trial has a processing status of Rejected.
- If the trial is complete and valid, the specialist accepts the submission. The trial has a processing status of Accepted.
- One or more CTRO Document Specialists perform abstraction on the Accepted trial, including administrative processing, scientific processing, and quality control. The trial has a processing status of Abstracted.
- The system sends the Trial Summary Report (TSR) of the Abstracted trial to the submitter. If appropriate, the system also sends an Abstracted XML file of the trial. (XML documents are available for trials that have been designated for import into ClinicalTrials.gov.) The trial has a processing status of Verification Pending.
- The submitter verifies the abstraction as per the TSR, and returns feedback to the CTRO within five business days after receiving the TSR. (A business day is any weekday that is not a Federal holiday. For a list of Federal holidays, refer to the U.S. Office of Personnel Management's list of Federal Holidays.)The trial has a processing status of Abstraction Verified Response.
- The CTRO Document Specialist decides, based on the feedback received, whether to restart administrative processing, scientific processing, or both for the trial (step 3).
- The CTRO Document Specialist completes the verification.
The following diagram illustrates the typical life cycle of a trial and the relationship between actions and processing statuses:
For more information, refer to Trial Processing Statuses and Amendment Process Life Cycle.
Role-Based Tasks in Registration
Each person who registers for a CTRP account is associated with a role. One registered user from an organization can request an Administrator role from the CTRO. That Administrator can grant administrative privileges to other users in the Administrator's organization.
CTRP account holders can perform only those tasks that are associated with their assigned roles.
The following table is a matrix of roles and tasks. A Yes or No in each table cell indicates whether someone with a given role can perform the task.
| Tasks | User | Site Administrator |
|---|---|---|
| Manage Accrual Access | No | Yes
|
| View Accrual Assignment History | No | Yes For Administrator's organization's or family member organization's trials. |
| Display Trial Ownership | No | Yes For any trial owned by the Administrator's site for which the organization is the lead organization. |
| Manage Trial Ownership | No | Yes |
| Manage Participating Site Record Ownership | No | Yes For registered users who are affiliated with the Administrator's organization. |
| Grant Administrative Authority | No | Yes For registered users who are affiliated with the Administrator's organization. |
| Manage Program Codes | No | Yes For details, refer to Managing Program Codes. |
| Request Administrative Authority | Yes | Yes |
| Manage System-Generated Emails | Yes Globally, for user's own account | Yes The system sends emails (including TSRs) to Site Administrators automatically only if the site Administrator's affiliated organization is the trial's lead organization, or if the Site Administrator is the trial submitter and/or trial owner. |
| Register Trials | Yes | Yes |
| Update/Amend Trials | Yes | Yes |
| Add/Update Participating Site Information | Yes | Yes |
Trial Categories (Study Sources)
Trials are categorized by type of Data Table 4 Funding Sponsorship or Trial Submission Category (study source).
- Complete Trials:
- National: NCI National Clinical Trials Network (NCTN) and other NIH-supported National Trial Networks.
- Externally Peer-Reviewed: R01s, SPORES, U01s, U10s, P01s, CTEP, or any other clinical research study mechanism supported by the NIH or organizations on this list: Organizations with Peer Review Funding Systems.
- Institutional: In-house clinical research studies authored or co-authored by Cancer Center investigators and undergoing scientific peer review solely by the Protocol Review and Monitoring System of the Cancer Center. The Cancer Center investigator has primary responsibility for conceptualizing, designing, and implementing the clinical research study and reporting results.
- It is acceptable for industry and other entities to provide support (such as drug, device, or other funding), but the trial should clearly be the intellectual product of the center investigator.
- This category may also include:
- Institutional studies authored and implemented by investigators at another Center in which your Center is participating.
- Multi-Institutional studies authored and implemented by investigators at your Center. (Note: National and externally peer-reviewed studies should be listed with those categories, not as Institutional studies.)
- Abbreviated Trials:
- Industrial: A pharmaceutical company controls the design and implementation of these clinical research studies.
- A CTRP organization family represents an NCI-designated Cancer Center family of organizations. For brevity, this guide refers to this entity as a Cancer Center family, a Cancer Center, or an organization family.
- A CTRP organization that is a member of a Cancer Center family is considered a Cancer Center organization. For brevity, this guide refers to this entity as a Cancer Center organization.
Creating CTRP Accounts
Although you already have an NIH (or NCI) account, you must register for an associated CTRP account. The CTRP account identifies your organization affiliation, whether or not you own a particular trial record, and other such details. You can create both types of accounts using Registration's account creation feature, in one of two ways, as follows:
- If you are a new user AND you do not have an NIH/NCI account, request one via Registration. Follow the instructions in Creating New CTRP Accounts via Email .
- If you are a new user AND you have an NIH/NCI account, use your current credentials to register for a CTRP account via Registration. Follow the instructions in Creating New CTRP Accounts Using NIH or NCI Credentials .
New users must have access to a valid email address to create an account.
Creating New CTRP Accounts via Email
If you are new to Registration and you do not have an NCI account, request a CTRP account via the Registration account creation feature.
Creating New CTRP Accounts Using NIH or NCI Credentials
Requesting Organization Admin Rights
You can request Admin rights for your affiliated organization to take advantage of the following features:
- Site administration - Accept or reject requests for Admin rights from other users within your organization
- Trial ownership management - Assign and unassign ownership of your organization's trials to other registered users within your organization
- Accrual access - Assign and unassign access to Accrual to registered users within your organization
- Program code management - Manage the set of program codes and program code assignments for your organization family. For details, refer to Managing Program Codes.
Condition for Admin Requests
The Request for Admin Access check-box on the My Account page is displayed only if no one else in your organization is an administrator.
How to Request Admin Rights
- On the top right corner of any page, click Your Username > My Account.
The My Account page displays all of your Registration account information. - Select the Request for Admin Access check box.
Managing Registration Email Notifications
The CTRP system can automatically send you an email message whenever some aspect of a trial you have submitted or own has changed. For example, the system would notify you when the CTRO has accepted a trial that you submitted. It would send another message when the Trial Summary Report (TSR) is available for review. You can choose to receive all messages or none of them.
How to Manage Registration Email Messages
- On the top right corner of any page, click Your Username > My Account. The My Account page displays all of your Registration account information.
- You have the following choices:
- To receive all system messages, set Receive Email Notifications to Yes.
- To opt out, set Receive Email Notifications to No.
- Click Save.