The Amend Trial page can be accessed by either of the following methods below:
Using the Trials to Verify menu
Using the Search menu
Using the Trials to Verify menu
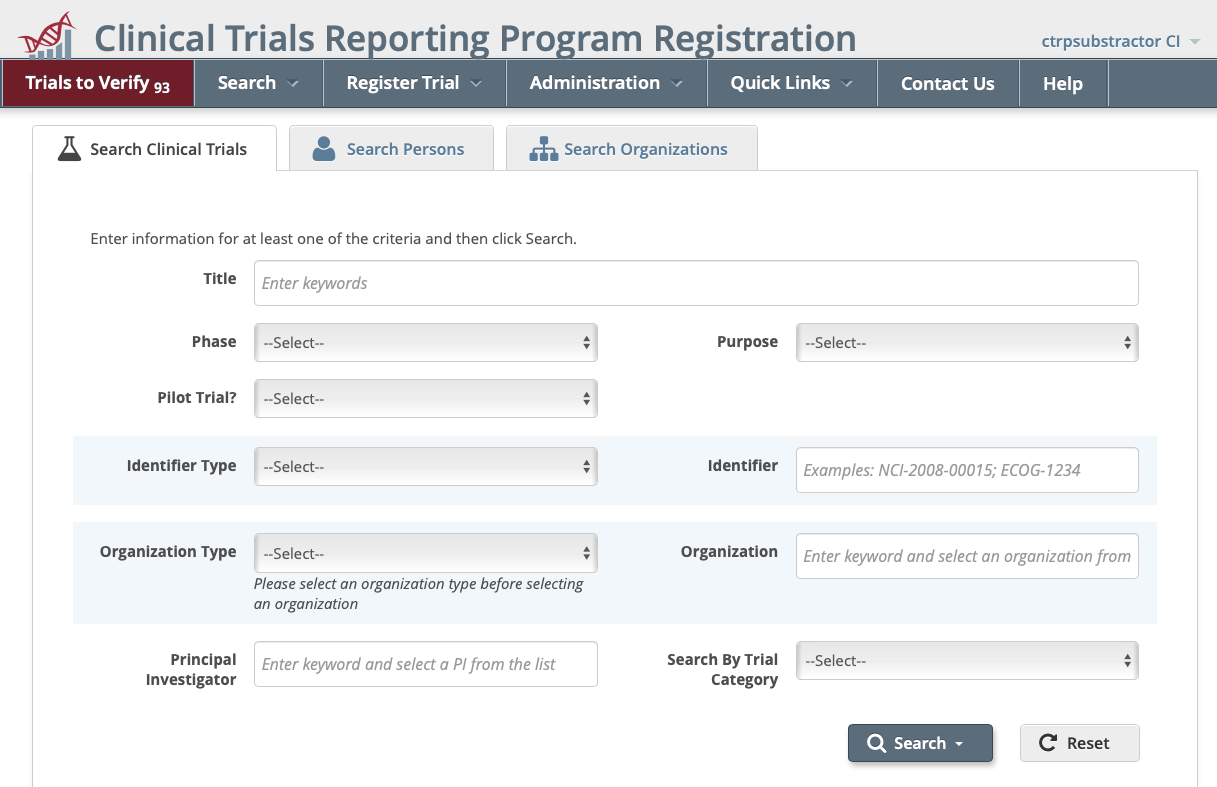
- Select the Trials to Verify menu from the main toolbar. The Trials Needing Verification page displays.
- Search for the trial in question by using the Search: box to perform a keyword search.
- Once the trial has been identified, perform one of the following actions:
- Click on the Identifier in the NCI Trial Identifier column:
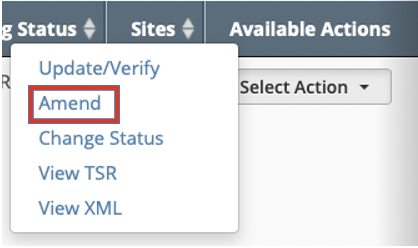
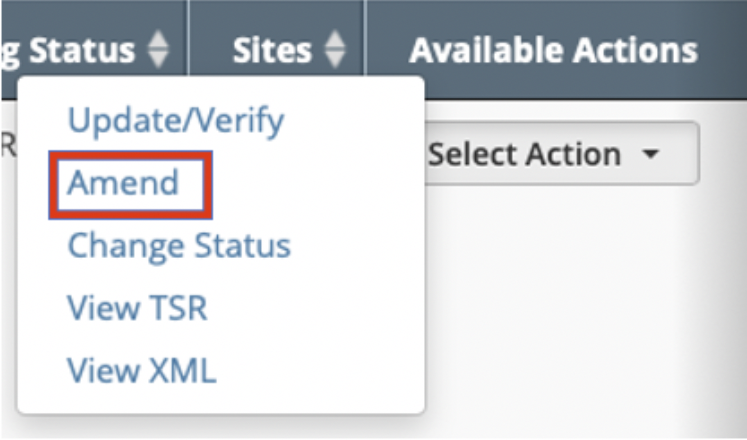
- Scroll horizontally to the Available Actions column and click Select Action > Amend.
- Click on the Identifier in the NCI Trial Identifier column:
Using the Search menu
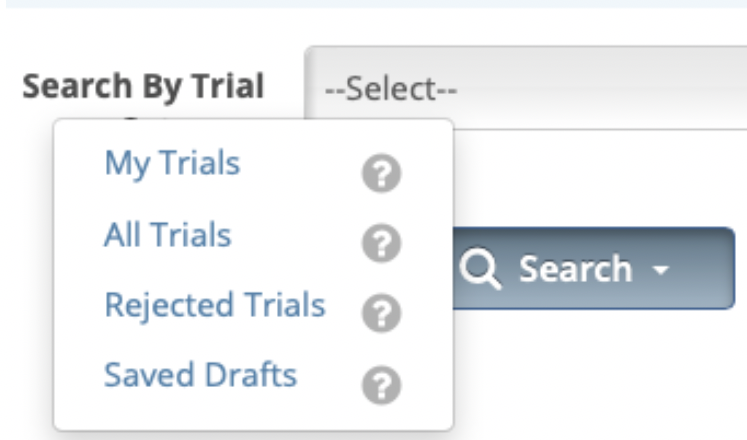
- From the Search menu, select Clinical Trials. The Search Clinical Trials page displays.
- Search for the desired trial(s) by using any of the filter options available on the page, or select the Search My Trials option from the Search button.
- On the Clinical Trials Search Results page, scroll horizontally to the Available Actionscolumn and click Select Action > Amend.
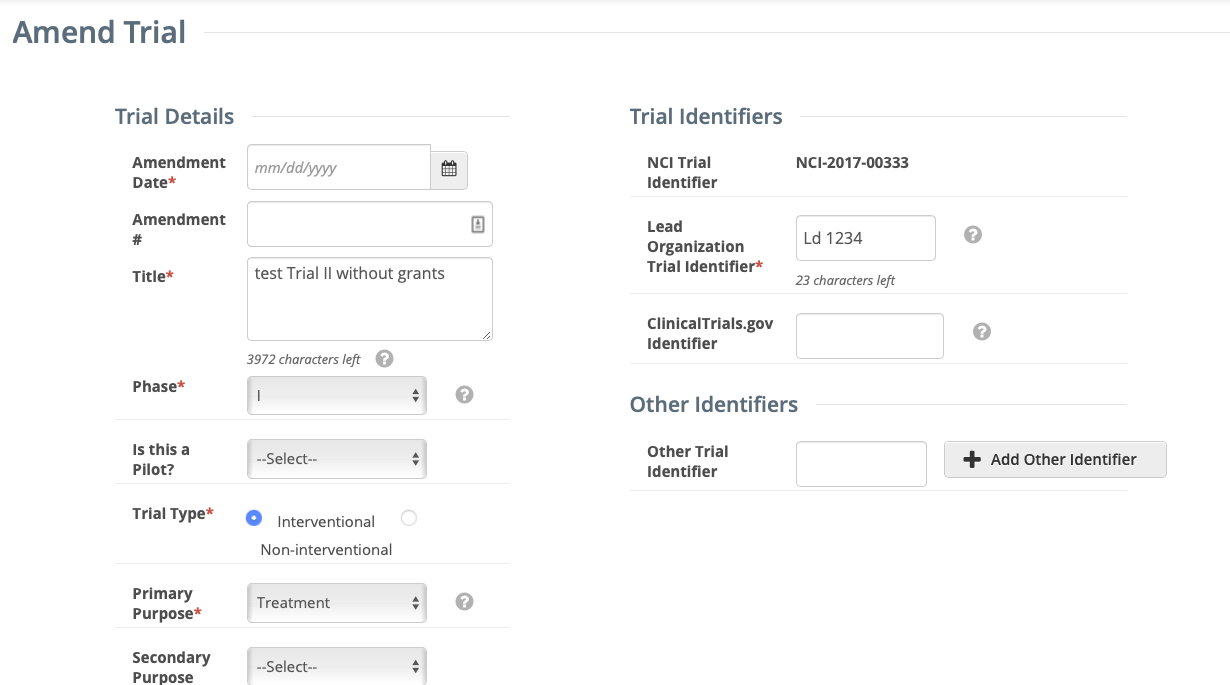
- The Amend Trial page displays the data currently registered with the CTRP.
Make changes to the fields as necessary. The system requires you to provide information for all fields marked with an asterisk (*). The instructions are the same for trial registration and trial amendment, with some exceptions:
In the Amendment Details section, specify the appropriate information in the various fields. The following table describes the fields.
Field Label Description/Instructions Amendment Number
Enter an appropriate number.
Amendment Date* Select or enter an appropriate date. You can select a different disease code only if accrual has not yet been reported to CTRP.
A trial can capture program codes from different organization families. For example, a participating site might belong to a different organization family than the lead organization. When you amend a trial, the Program Code field displays all codes from the master list for the organization family of the lead organization.
Primary Completion Dates are optional for non-interventional trials. The system excludes such trials when submitting XML documents to ClinicalTrials.gov. Otherwise, Primary Completion Dates are required.
The system does not change the status of participating sites when you close a trial.
- For instructions on recording each field otherwise, refer to Registering New Trials.
Submitting Amendments
- Scroll to the bottom of the Amend Trial page, and click Submit Amendment.
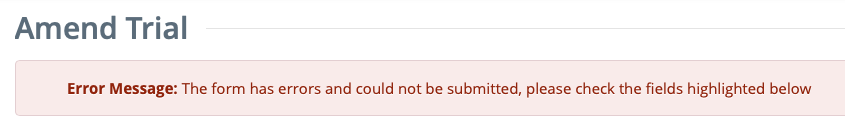
The system checks for errors and missing information.- If errors are found: An Error Message is displayed at the top of the Amend Trial page.
Correct any errors if indicated, and re-submit the amendment as many times as necessary until the amendment is error-free.
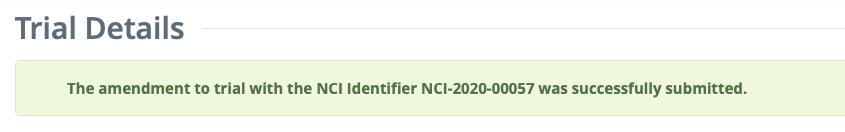
If no errors are found: The Trial Details page is loaded with a confirmation message at the top of the page:
- If errors are found: An Error Message is displayed at the top of the Amend Trial page.
- The system sends you an email notification — with the details of what has changed — whenever you amend trials.