You can view the Trial Summary Report (TSR) for Complete trials you own.  The View TSR link is available even if the CTRO has not accepted the trial. |
You can view the XML document for a trial you own if it meets the following criteria: - Trial category is Complete
- Processing status is one of the following:
- Abstraction Verified Response
- Abstraction Verified No Response
- On Hold (if the trial was put on hold after it reached either of the two statuses above)
- XML Required, Enable "Upload from NCI CTRP in ClinicalTrials.gov?" indicator is Yes
How to View TSR and XML Documents
-
 Search for the trial(s) for which you want to view a TSR or XML document. Search for the trial(s) for which you want to view a TSR or XML document.
The Submitted Clinical Trials Search Results table displays the trial(s) you searched for. Available actions are listed (if any) for each record.
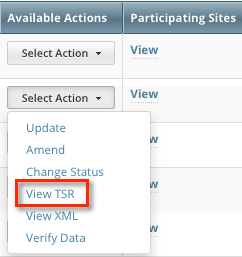 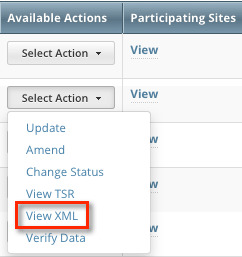 - In the Available Actions column, click Select Action > View TSR or Select Action > View XML.
|