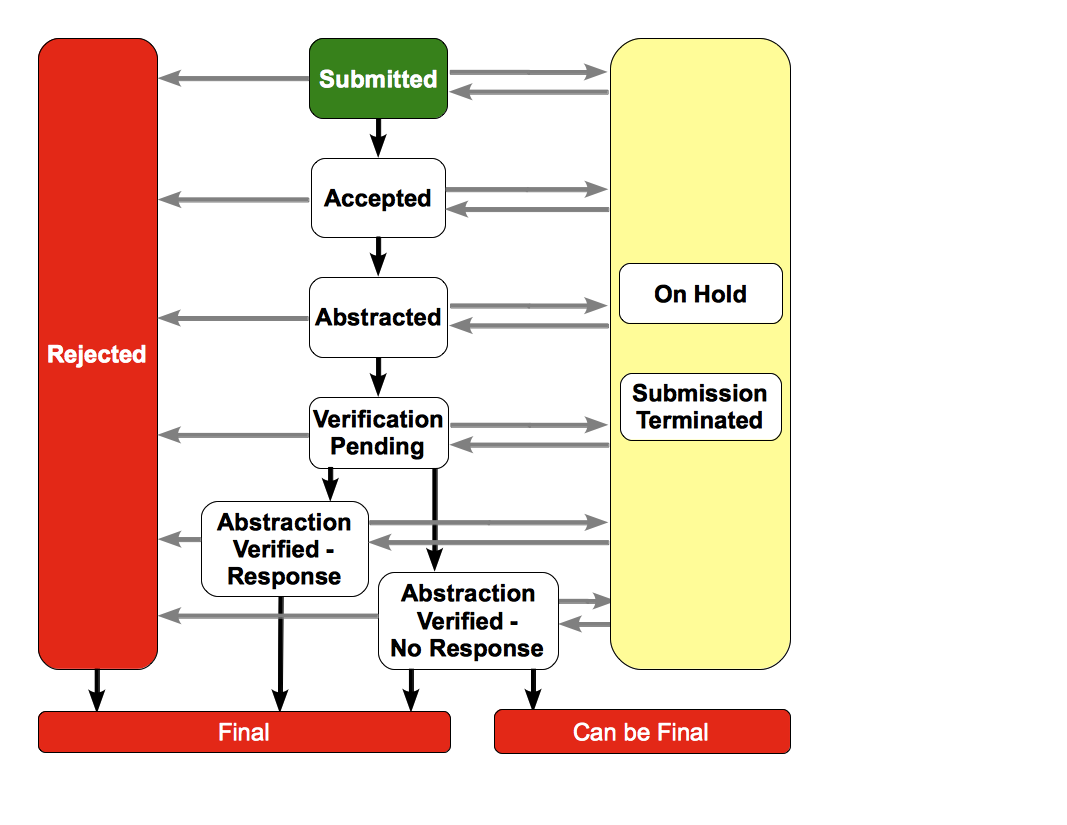
Your ability to record milestones depends on trial processing statuses. The following diagram illustrates the basic workflow of trial processing statuses. The rules that follow it provide more detailed information about the order in which you can record the milestones.
The following rules determine the order in which you can record the milestones:
- You can record most milestones multiple times per trial, following the order of the trial submission processing life cycle. However, you can add the following milestones only once per trial submission:
- Submission Received Date
- Submission Rejection Date
- Initial Abstraction Verified Date
- Initial Submission to ClinicalTrials.gov
- You cannot record milestones with a future date.
- Each milestone date must follow the preceding milestone date.
If the most current processing status is this... | You can record this milestone |
|---|---|
Submitted | Submission Received Date |
Accepted and all Administrative and Scientific abstraction activities have been completed | QC Completed Date. Recording this milestone sets the trial processing status to Abstracted and Trial Verification Date to the date that you record this milestone. |
Verification Pending and a Submitter TSR Feedback Date milestone has been recorded | Initial Abstraction Verified Date. |
Verification Pending and a Submitter TSR Feedback Date milestone has not been recorded | Initial Abstraction Verified Date. |
Abstraction Verified Response | On-going Abstraction Verified Date |
Abstraction Verified No Response | On-going Abstraction Verified Date |
Abstracted | Trial Summary Report Date. |
Submission Terminated | Submission Reactivated Date. This is the only milestone that you can record after the Submission Terminated Date. |
If the most current processing status is this... | You can not record this milestone |
|---|---|
Submitted | Trial Summary Report Date |
Accepted | Trial Summary Report Date |
This date... | Must precede this date |
|---|---|
Ready for Administrative QC Date | Administrative QC Start Date |
Ready for Scientific QC Date | Scientific QC Start Date |
Administrative QC Start Date | Administrative QC Completion Date |
Scientific QC Start Date | Scientific QC Completion Date |
TSR Date | Submitter TSR Feedback Date |
Administrative Processing Start Date | Administrative Processing Completed Date |
Scientific Processing Start Date | Scientific Processing Completed Date |
Submission Terminated Date | Submission Reactivated Date |
You can not record this milestone... | If this is true |
|---|---|
QC Start Date |
|
Trial Summary Report Date |
|
Submitter Trial Summary Report Feedback Date |
|
Initial Abstraction Verified Date |
|
Initial Submission to ClinicalTrials.gov |
|
On-going Abstraction Verified Date |
|
Late Rejection Date |
|
Ready for QC Date |
|
Submission Terminated Date | The trial has been rejected |