Trials are conducted in participating sites. Information about the site (organization), investigator(s), and primary contacts (if a central contact is not provided) must be abstracted. Information about participating sites can be included in the protocol document or in the Participating Sites document.
The system assigns investigators and primary contacts a status code that corresponds to the person’s/role’s curation status.
Abstracting Participating Site Information
Search for the trial of interest. For instructions, refer to Searching for Trials in PA.
In the search results, select the NCI Trial Identifier link for the desired trial. This will open the Trial Identification page.
On the Trial Identification page, check out the trial. For instructions, refer to Checking In and Checking Out Trials. (This checkout step is optional for Super Abstractors.)
On the Administrative Data menu, select Participating Sites.
On the Participating Sites page, select Add.
The Participating Sites page displays three tabbed sections: Participating Site, Investigators, and Contact.
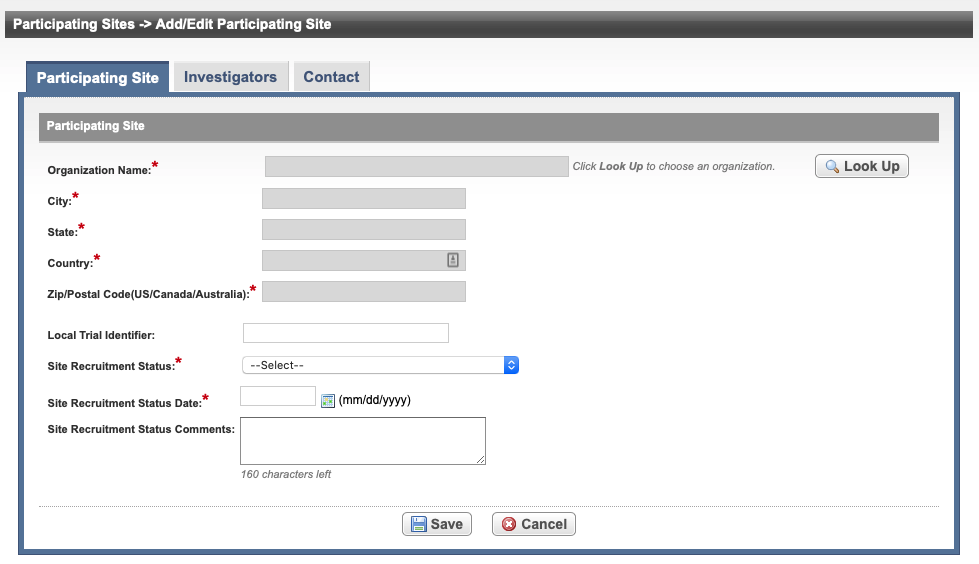
Add/Edit Participating Site- Complete Trial
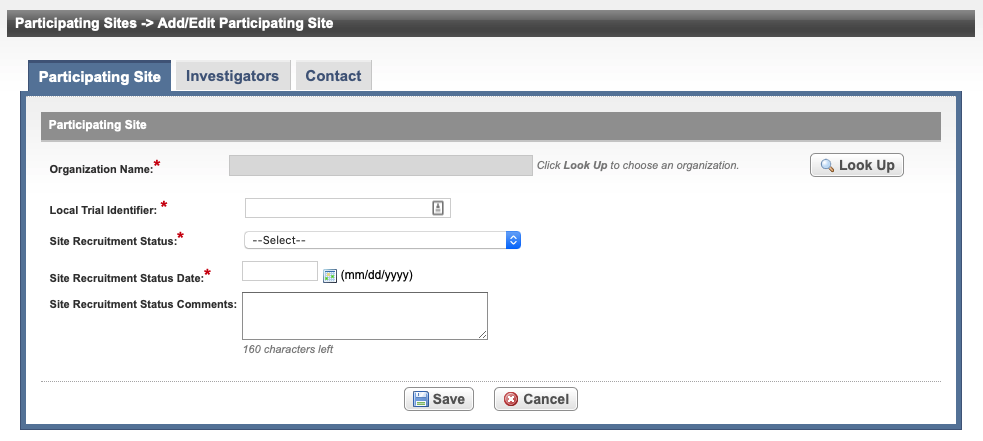
Add/Edit Participating Site - Abbreviated Trial
See the following table for reference on the Participating Site fields. An asterisk (*) indicates a required field.
Field Label
Description/Instructions
Organization Name*
Select Look Up and follow the instructions in Searching for Organizations. For Complete trials, the City, State, Country, and Zip/Postal Codes fields are populated when an organization is selected.
Local Trial Identifier*
Enter the site trial identifier.
Site Recruitment Status*
- Select the status from the drop-down list. Refer to Trial Status Values in the CTRP and ClinicalTrials.gov and Expanded Access Statuses for additional details regarding
CTRP validates all status transitions when a trial status record is saved. If a status transition is added or updated which does not conform to the rules provided in Trial Status Transitions, CTRP displays errors and/or warnings. Warnings indicate that correcting the transition in the trial status record is optional. However, Errors indicate that correcting the transition in the trial status record is required. All transitions can be updated in the Participating Sites Status History window. The trial cannot be checked in until all status transition errors have been resolved. For a comprehensive matrix of valid transitions, see Trial and Participating Sites Status Transition Rules.
Site Recruitment Status Date*
Enter the date that the status was recorded. The date must be the current date or earlier.
Site Recruitment Status Comments Enter one or more comments about the site recruitment status. Select Save.
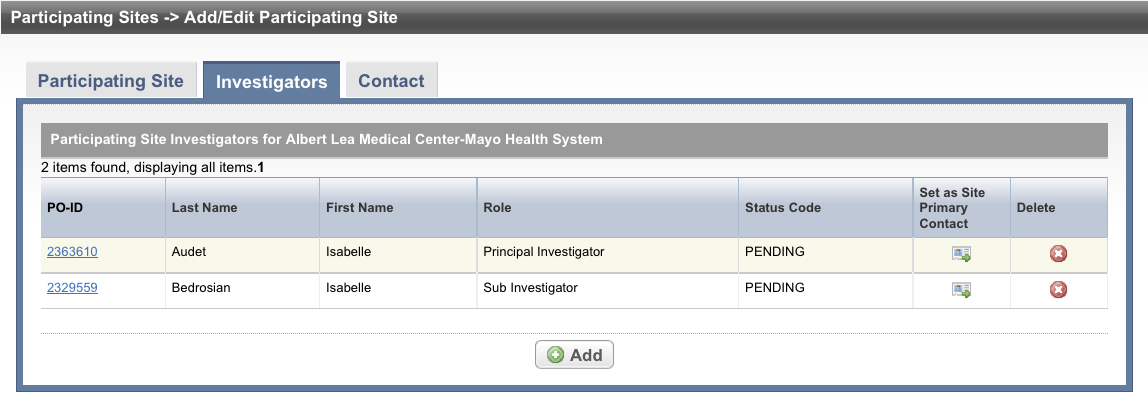
Select the Investigators tab. The Investigators tab displays the trial investigators that may have been added during trial submission or abstraction.
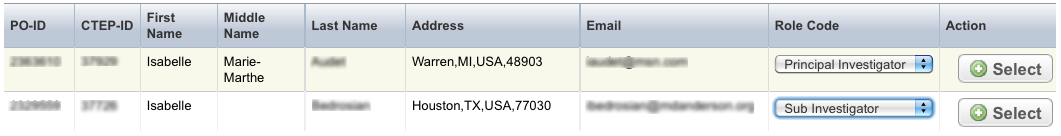
- Select Add and search for the investigator’s name by following the instructions in Searching for Persons.
- When you find the investigator in the search results list, assign the investigator role, either Principal Investigator or Sub Investigator, and then click Select. The investigator selected will be added to the Investigators tab.
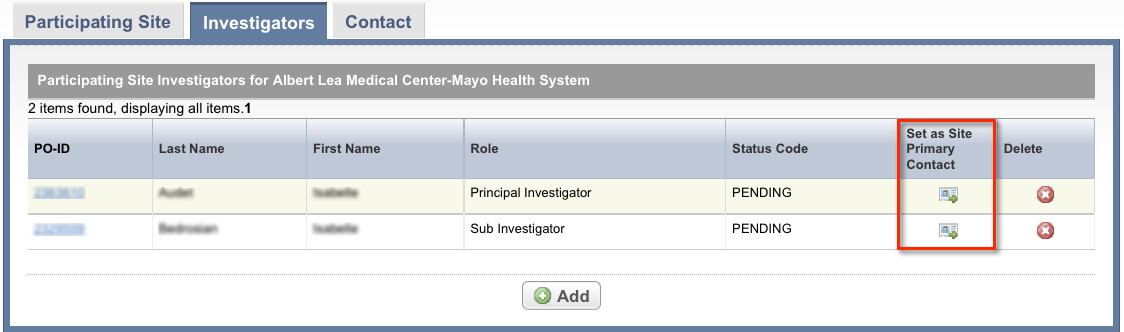
To indicate that an investigator is the primary contact, select the Set as Site Primary Contact icon next to this investigator's record.
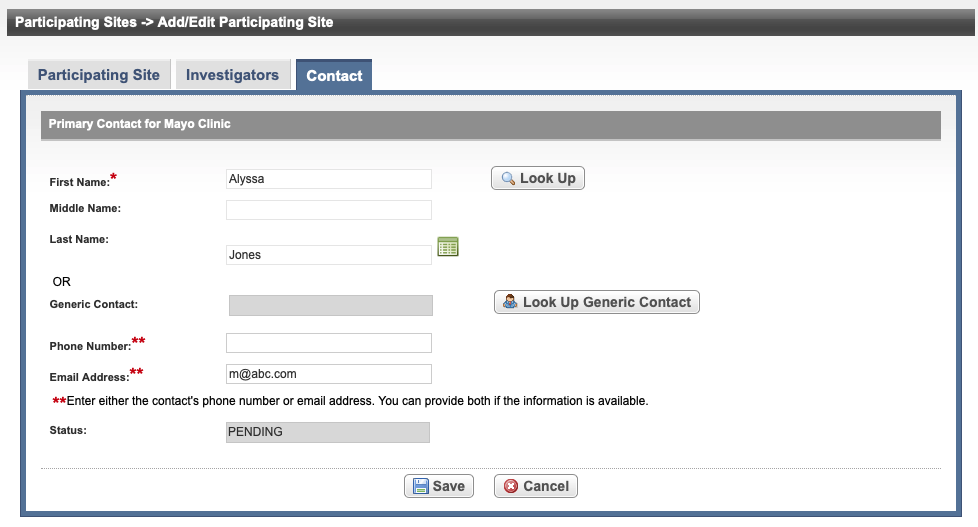
Select the Contact tab.
A Participating Site must be linked to the trial before abstracting the site PI and contact information. A contact can be added by providing a person’s name (i.e., someone who is associated with the trial itself), or a generic contact can be added (i.e., someone who is associated with the site but not necessarily the trial) by providing a person’s title (functional role). Both types of contacts can not be added in the same record. An abstraction cannot be completed if a primary contact is not assigned.
If an investigator is designated as the primary contact (on the Investigators tab), the investigator's name is displayed on the Contact tab.- On the Contact tab, next to the First Name field, select Look Up and search for the contact person’s name by following the instructions in Searching for Persons.
In the various fields, specify the appropriate information. The following table describes the fields. An asterisk (*) indicates a required field.
Field Label
Description/Instructions
Phone Number*
Enter the contact’s primary telephone number (as 123-456-7890), including an extension if provided.
*Either the contact's phone number or email address are required. Both can be provided.
Email Address*
Type the contact’s primary email address.
*Either the contact's phone number or email address are required. Both can be provided.
Status CTRP populated after the record is saved. - Select Save.