After you establish that all trial information is correct, accept the trial to make it available for future abstraction.
- If the trial contains incorrect information that you can not modify or verify, place the trial on hold. See Putting Trial Processing On Hold for instructions.
If the trial is a duplicate of another in the CTRP database, or is invalid, reject the trial.
If you determine that a trial is an invalid submission, search for valid submissions in the CTRP database.
Before you accept a trial, verify the following trial characteristics:
Distinctiveness. 1 Verify that the trial is not a duplicate of one previously registered and accepted in the CTRP.
Appropriateness. For example, the CTRO accepts only trials submitted by a lead organization, and not a participating site in the case of a multi-site trial with an existing coordination center.
- Completeness. For example, if a given type of document is missing, verify that it is included in another trial document.
- Accuracy. For example, verify that the principal investigator’s information is correct.
- Consistency. For example, verify that the Protocol document is consistent with the submitted trial details.
All required fields must contain information in order to be accepted.
--
- The CTRP system checks for duplicate trials by ClinicalTrials.gov identifier. However it does not check for duplicate titles or other aspects of the trial. If you find a duplicate trial, decide which is correct as per the Protocol document, and then reject the other. The system displays an error message if it detects that there is an existing trial with the same ClinicalTrials.gov ID when you click Save on the General Trials Details page during validation. In order to save the trial information successfully, you must first change the ClinicalTrials.gov ID. ↩
How to Accept and Reject Trials
- Select a trial to validate by following the instructions in Selecting Trials that Require Validation. The Trial Identification page displays the trial details.
- On the Trial Identification page, check out the trial. For instructions, see Checking In and Checking Out Trials.
- On the Validation menu, click Trial Validation. The Trial Validation page displays trial details.
To accept the trial, click Accept. A message confirms acceptance, the trial processing status changes to Accepted, and the system sends a notification of acceptance email message to the trial submitter. Also, the abstraction menu appears with options specific to abstracting the trial.
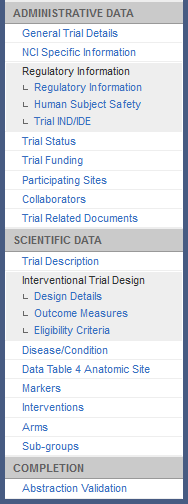
Abstraction Menu for Interventional Trials
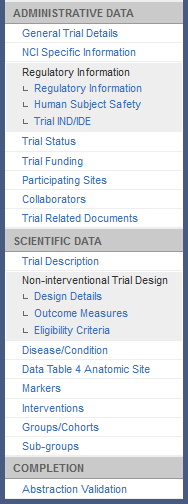
Abstraction Menu for Non-interventional Trials
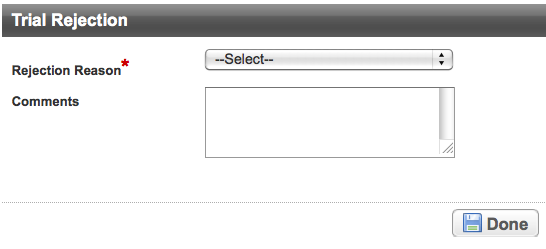
To reject the trial, click Reject.
The Reason For Rejection section appears.When you reject a trial that is on-hold, the system takes the trial off hold.
- In the Reason For Rejection field, type the reason for rejecting the trial. For example, if you rejected the trial because you found a duplicate record in the system, enter
Duplicate of record NCI-2008-1234. - Click Done.
A message confirms rejection. The trial processing status changes to Rejected, and the system sends a notification of rejection email message to the trial submitter.
Rejecting Amended Trial Submissions
After you reject an amendment, the system verifies the rejection in a message and returns you to the Trial Identification page. The Last Submitter, displayed in the heading across the top of the page, reverts to the person who submitted the trial prior to the amendment submission.