The FDA has mandated sponsors whose studies start after Dec 17, 2016, must submit their clinical study data sets in the Study Data Tabulation Model (SDTM) standard format. For INDs, the requirement applies for studies that start after Dec. 17, 2017. SDTM provides a standard for organizing and formatting data to streamline the process in collection, management, analysis and reporting. The Clinical Data Interchange Standards Consortium (CDISC) is a global nonprofit standards development organization with a worldwide team of staff and volunteer experts across the medical community. CDISC provides data standards to streamline clinical research, one of which being the SDTM. CDISC is also developing Clinical Data Acquisition Standards Harmonization which establishes a standard way to collect data in a similar way across studies and sponsors so that data formats and structures provide clear tractability of submission into the SDTM.
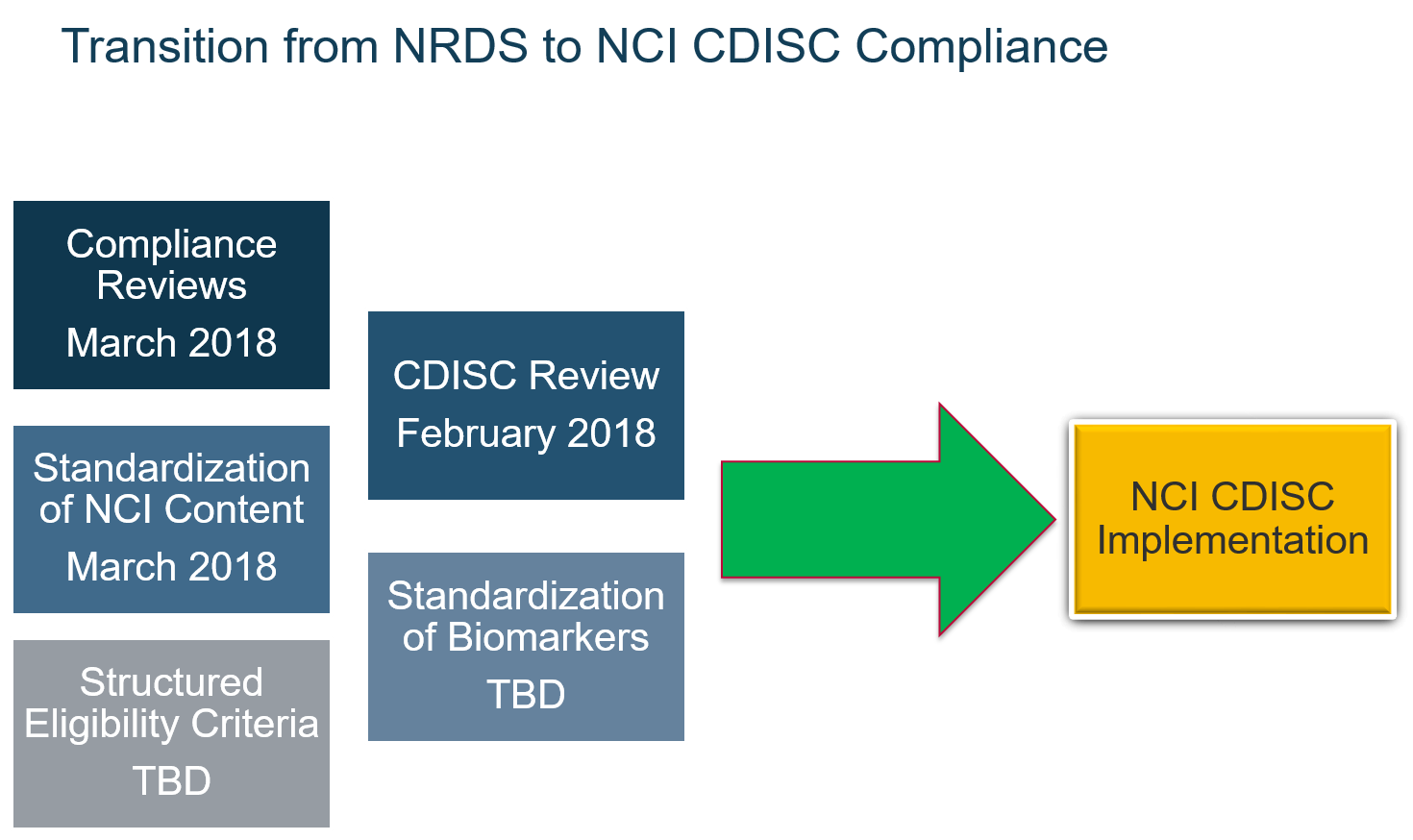
To support the FDA mandate of submitting clinical study stat sets to the FDA in the SDTM format, the NCI is transitioning their current Network Rave Data Standards (NRDS) Initiative, led by the Cancer Therapy Evaluation Program (CTEP) into the CDISC implementation.
Quick Links