This page provides an overview of the changes in CTRP. For any questions or issues regarding these feature enhancements, please contact the CTRO (NCICTRO@mail.nih.gov).
September 2020
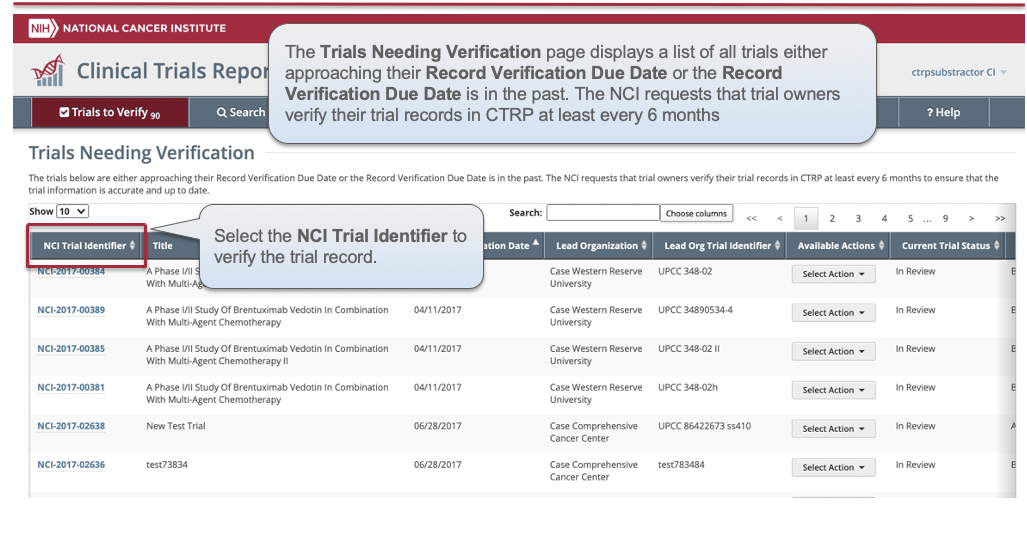
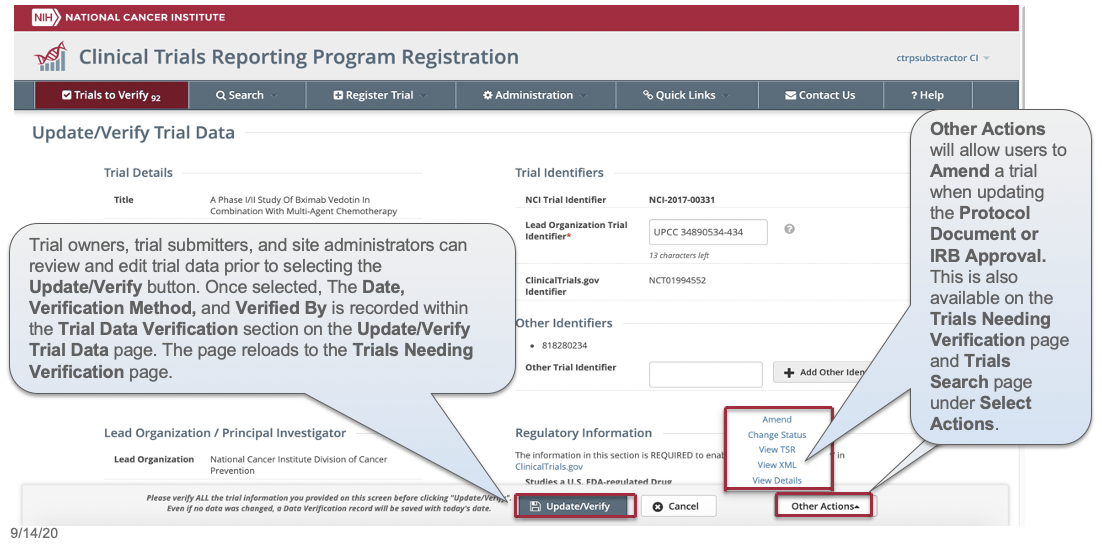
CTRP Trial Record Verification: Improved the user experience for CTRP trial owners, trial submitters and site administrators with Verifying, Updating and/or Amending their trial records. Key highlights from this enhancement include:
- New page highlighting all Trials Needing Verification
- Ability to view all trial details on the Verify screen
- Simplified workflow allowing users to Verify, Update or Amend trials within same form
- New monthly email notification for alerting trial owners, trial submitters,
and site administrators of Trials Needing Verification
For more information on the CTRP Trial Record Verification processes, please visit these CTRP User Guide pages:
https://wiki.nci.nih.gov/display/CTRPdoc/Updating+Trial+Information
https://wiki.nci.nih.gov/display/CTRPdoc/How+to+Verify+Trial+Data
https://wiki.nci.nih.gov/display/CTRPdoc/How+to+Access+the+Trial+Data+Verification+Page
https://wiki.nci.nih.gov/display/CTRPdoc/Amending+Trials
July 2020
Flexible Accrual: Enhanced the CTRP accrual reporting capabilities to include more “Flexible Accrual” reporting options. Centers can now request to update the default accrual reporting type on a study registered in CTRP. Changing the default accrual reporting type requires a ticket to be submitted to the CTRO (NCICTRO@mail.nih.gov) for review and approval, before any change is granted.
The current default accrual reporting types are as follows:
Trial Type
Default Accrual Reporting Type
Complete Interventional
Subject
Complete Non-Interventional
Subject
Abbreviated/Imported Interventional
Summary
Abbreviated/Imported Non-Interventional
Summary
- CTRP is also introducing a new accrual reporting type, “Partial Subject.” Partial Subject accrual requires the reporting of Study Subject ID, Registration Date and Participating Site data only. The other accrual data fields are optional. The reporting of Partial Subject accrual also requires a ticket to be submitted to the CTRO (NCICTRO@mail.nih.gov) for review and approval, before any change is granted.
- Accrual Data Element changes:
- Study Subject Birth Date: For any/all ways to report accrual, only the month and year (MM/YYYY) are required to be collected.
- Study Subject Country: Required for all methods of reporting accrual (was previously optional)
- Disease:
- Study Subject: Required
- Partial Subject: Not required. However, for ICD-O-3 Disease Codes if Disease is reported, then Site must be reported.
- Site:
- Study Subject: Required
- Partial Subject: Not required. However, for ICD-O-3 Disease Codes if Site is reported, then Disease must be reported.
- Disease Codes: CTRP uses a hybrid model with the codes from IACR and the codes in NCI SEER that are not available in IACR.
For more information on the CTRP Accrual process, including Flexible Accrual, Partial Subject accrual and other changes made within the Accrual application (e.g., ability to Delete All Trial Study Subjects,) please visit these CTRP User Guide pages:
https://wiki.nci.nih.gov/display/CTRPdoc/About+Accrual
https://wiki.nci.nih.gov/display/CTRPdoc/Searching+for+Trials+in+Accrual
If you would like to receive future CTRP announcements you may add yourself to the list here: https://list.nih.gov/cgi-bin/wa.exe?SUBED1=ctrp-users-l&A=1