Validate participating sites for Abbreviated trials.
Participating sites validation does not pertain to Complete trials.
Ensure that the following information is complete and accurate:
- Organization Name
- Local Trial Identifier
- Site Principal Investigator
- Site Recruitment Status
- Site Recruitment Status Date
How to Validate Participating Sites
- Select a trial to validate by following the instructions in Selecting Trials that Require Validation. The Trial Identification page displays the trial details.
On the Trial Identification page, check out the trial. For instructions, refer to Checking In and Checking Out Trials. (This checkout step is optional for Super Abstractors.)
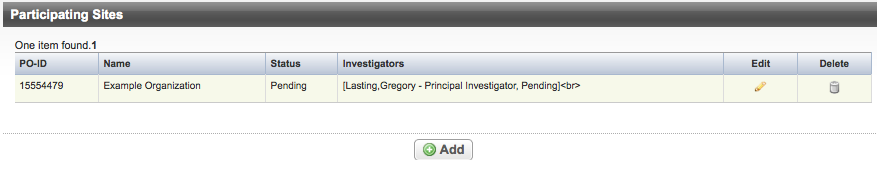
- On the Validation menu, click Participating Sites.
The Participating Sites Page appears.
- Do one of the following to continue:
- To add participating sites, click Add and follow the instructions provided in Abstracting Participating Sites.
- To modify an existing record, click the Edit icon and follow the instructions provided in Abstracting Participating Sites.
- To delete a record, click the Delete icon.
- If you made any changes, click Save.