|
Page History
- For instructions information on how to access the Update/Verify Trial Data page (for Complete trials), or the Update Abbreviated Trial page (for Abbreviated/Imported trials) see the following: How to Access the Update/Verify Trial Data Page
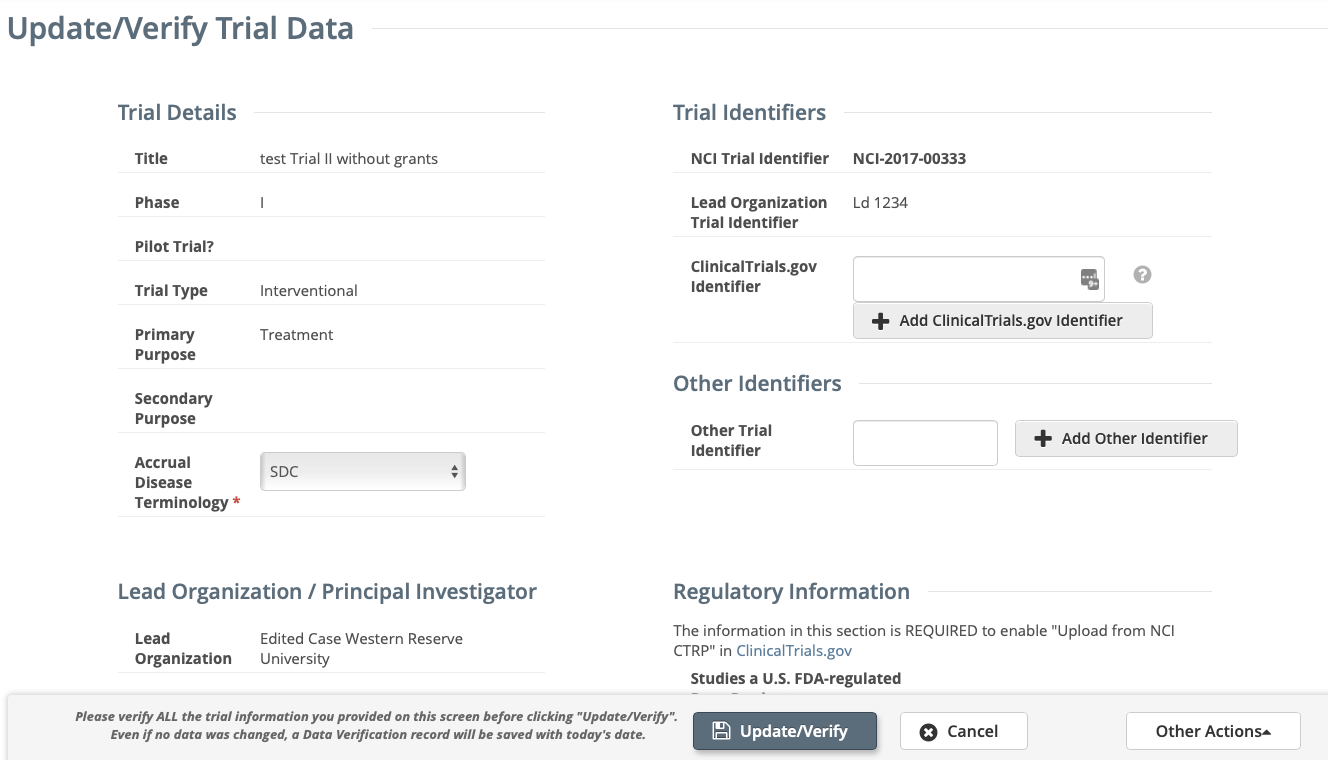
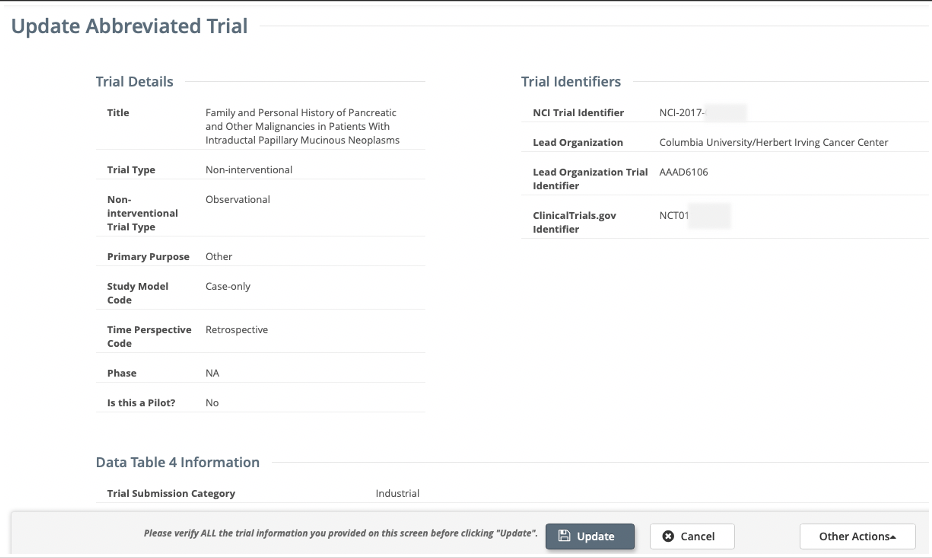
- Review the information on the Update/Verify Trial Data page or Update Abbreviated Trial page for accuracy, update available fields as necessary.
. Required fields are marked with an asterisk (*). For data changes on fields that are not editable, contact the CTRO at NCICTRO@mail.nih.gov.
Update/Verify Trial Data page for Complete trials
Update Abbreviated Trial page for Abbreviated/Imported trials
If applicable, enter a ClinicalTrials.gov Identifier, and then click Add ClinicalTrials.gov IdentifierNote An existing ClinicalTrials.gov Identifier cannot be changed. If the ClinicalTrials.gov Identifier specified for a trial is incorrect, contact the CTRO at NCICTRO@mail.nih.gov.
Info When a trial is submitted, the system validates the NCT ID to ensure that the ID is unique among all other registered trials in CTRP. If a duplicate trial is found, an error message is displayed. If this occurs, verify the NCT ID. If you are certain that the number you entered is correct, contact the CTRO at NCICTRO@mail.nih.gov.
Additional Notes:A trial can capture program codes from different organization families. For example, a participating site might belong to a different organization family than the lead organization. When you amend a trial, the Program Code field displays all codes from the master list for the organization family of the lead organization.
Primary Completion Dates are optional for non-interventional trials. The system excludes such trials when submitting XML documents to ClinicalTrials.gov. Otherwise, Primary Completion Dates are required.
- If appropriate, upload any new or updated documents. For instructions, refer to Uploading Trial-Related Documents. If you upload an IRB document, the CTRO reviews the updated record you submit and makes changes to the record as necessary. For example, if you upload an IRB document for a trial currently in the In Review state, the CTRO updates the IRB information section of the trial record (e.g., IRB status and approval number).
For instructions on recording each field otherwise, refer to Registering New Trials.
- Select the Update/Verify button (for Complete trials) or Update button (for Abbreviated/Imported trials).
One of the following verification messages will display depending how the Update/Verify Trial Data or Update Abbreviated Trial page was accessed:
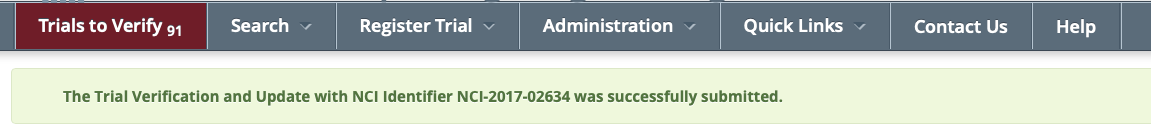
Trials to Verify menu: The Trials Needing Verification page
Note:will reload, the count on the Trials to Verify tab will decrease by 1 and the following confirmation message will be displayed:
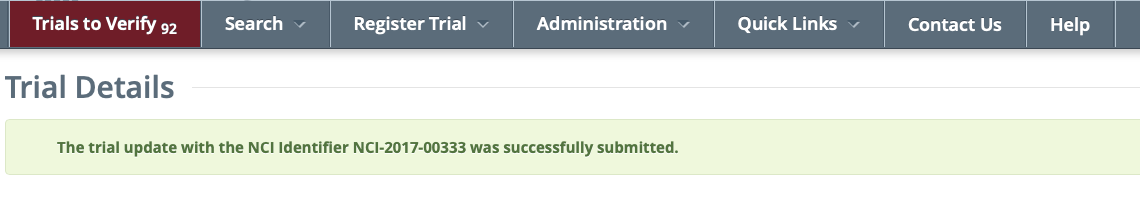
Search menu: The Trial Details page will reload and the following confirmation message will be displayed:
| Info | ||
|---|---|---|
| ||
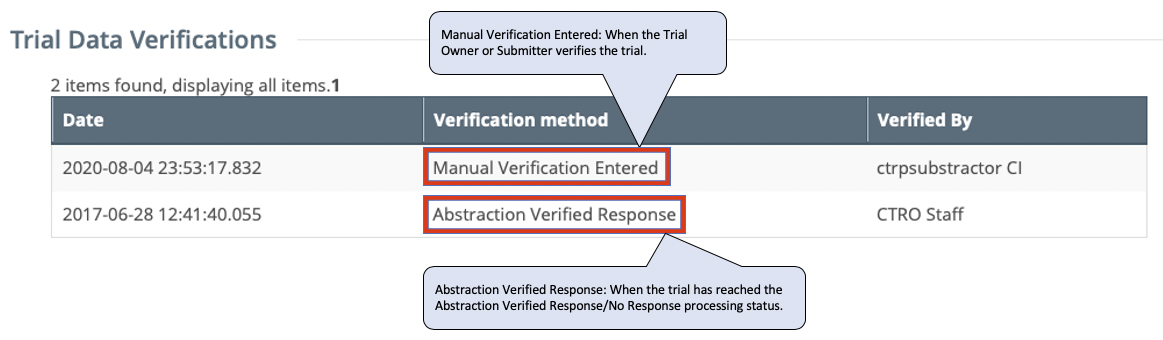
To review the updated verification record, return to the Trial Details or Update/Verify Trial Data |
...
page (Complete trials) for the trial. The Trial Data Verifications section displays all verification records to date. |