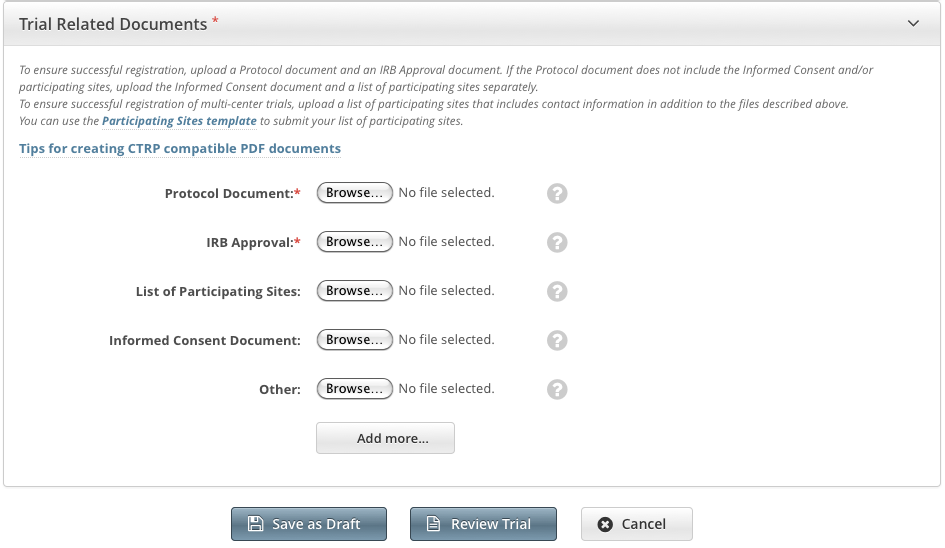
When registering Complete trials, you must upload the following types of documents:
- Complete clean Protocol document
- IRB Approval
- List of Participating Sites (if not included in the protocol document). Multi-site trials require a list of participating sites and contact information.
Informed Consent (if not included in the protocol document)
Currently you are required to supply your documents as Microsoft Word (.doc, .docx, or .docm), Adobe PDF, Microsoft Excel (.xls, .xlsx, .xlsm, or .xlsb), and/or WordPerfect files.
Special processing for PDF files
Adobe PDF files require special processing. See the information about creating PDFs in Converting Trial-Related Documents to PDFs.
How to Submit Trial Related Documents
- Next to the document-type field, (e.g., Protocol Document), click Browse.
Navigate to, and select, the appropriate document, and then click Open.
Depending on your operating system, you may see a different command name for "Open."
Repeat the steps above for each type of document.
Adding Multiple "Other" Documents
You can upload more than one (1) "Other" document. After you have uploaded the first of your "Other" documents, click the Add More link. A new Other document field is displayed.