ISA-TAB Specification
ISA-TAB-Nano extends ISA-TAB, an existing specification developed by the ISA-commmunity. Please refer to the ISA-TAB Specification for guidelines on the format of ISA-TAB.
ISA-TAB-Nano OVERVIEW PRESENTATION - a great place to start!
ISA-TAB-Nano Paper: BMC Biotechnology 2013, 13:2 Published: 14 January 2013
Commentary Nature Nanotechnology 2013, 8, 73-74 Published: 5 February 2013
ISA Model and Serialization Specifications
ISA-TAB-Nano: Select each File type template: Investigation | Study | Material | Assay
See each File page for more information and links to examples and glossaries.
Scope
This standard (ISA-TAB-Nano) specifies the format for representing and sharing information about nanomaterials, small molecules and biological specimens along with their assay characterization data (including metadata, and summary data) using spreadsheet or TAB-delimited files.
Prerequisites
Familiarity with the fields of nanotechnology and nanomedicine is a prerequisite for this specification. An understanding of ISA-TAB is recommended but not required as the ISA-TAB-Nano specification provides descriptive information on ISA-TAB as applied to nanotechnology.
Version
The current version of ISA-TAB-Nano is Version 1.3 (March 2020). For differences between Version 1.3 and 1.2, refer to the ISA-TAB-Nano 1.3 Release Notes.
ISA-TAB-Nano Introduction
Rationale
The field of nanomedicine faces many challenges in the development of standards to support meaningful data submission and information exchange. Nanomaterial characterization requires numerous physico-chemical, in-vitro, and in-vivo assays where measurements mostly depend on non-standardized protocols and diverse technology types. Unfortunately, information describing the nanomaterial, including functionalizing entities and three-dimensional (3D) structure, is often represented in an undisciplined fashion. In addition, there has been no standard way to associate this information with the data and metadata from characterization studies. This lack of standardization has been a significant deterrent to meaningful data sharing across the nanotechnology community; few publications contain sufficient information to enable adequate interpretation of results and successful achievement of experimental reproducibility. Furthermore, there has been very limited success in using non-standardized data to represent or derive structure-activity-relationships (SARs) that are critical for understanding the effects of nanomaterial structure on biological activity in nanomedicine.
Significance
The ISA-TAB-Nano specification is intended to facilitate the submission and exchange of nanomaterial descriptions and characterization data (metadata and summary data) along with the other files (raw/derived data files, image files, protocol documents, etc.) among individual researchers and to/from nanotechnology resources like the NCI’s cancer Nanotechnology Laboratory (caNanoLab) portal and the Nanomaterial-Biological Interactions (NBI) knowledgebase . ISA-TAB-Nano also serves to empower organizations to adopt standard methods for representing data in nanotechnology publications; and to provide researchers with guidelines for representing nanomaterials and characterizations to achieve cross-material comparison.
ISA-TAB-Nano Development
The ISA-TAB-Nano project is an effort of the National Cancer Institute (NCI) Nanotechnology Working Group (Nano WG).
ISA-TAB-Nano Standards Re-Use
ISA-TAB
The ISA-TAB-Nano format specification is based on an existing specification developed by the ISA-community , namely, the investigation/study/assay (ISA-TAB) format specification. The ISA-TAB format is used by the ‘omics’ (proteomics, genomics, metabolomics, and transcriptomics) communities to share data and metadata associated with different assays and technology types in their experiments. The ISA-TAB file structure relies on three primary files---investigation, study, and assay (ISA) files. Raw/derived data files and any other files (such as image files or protocol documents) specific to each assay are shared along with the three primary ISA-TAB files if the data files are referenced in the primary ISA-TAB files. ISA-TAB does not provide format specification for files other than the investigation, study, and assay files. The ISA-TAB investigation file has three purposes: (1) to record all declarative information referenced in other files; (2) to relate assay files to study files; and (3) to group multiple study files that are part of the same investigation. The ISA-TAB study file records information about the source, sampling methodology, treatment, preparation, and characteristics of the subjects (biospecimens) studied using one or more assays under an investigation.
National Cancer Institute Enterprise Vocabulary Service (NCI EVS)
The NCI EVS is a project of the NCI Center for Biomedical Informatics and Information Technology (CBIIT). EVS provides controlled terminologies and ontologies in support of the biomedical research and informatics activities of the NCI and its partners. The activities of the EVS include development of terminologies, development of terminology-related software, and operations support to address the broad spectrum of terminology needs in the cancer research enterprise. Among the vocabularies that EVS supports is the NanoParticle Ontology (NPO), by providing terminology development facilities and terminology servers, which are made available both via the web and programmatically through EVS server APIs. Additionally, the NPO is presented to the public by EVS both in a standalone format and as a component of the NCI Metathesaurus, where its concepts are mapped to the concepts of other vocabularies used by the NCI community. Also, the EVS-managed NCI Thesaurus (NCIt) includes nanotechnology concepts that have been utilized in the development of the NCI caNanoLab. Data from caNanoLab has been utilized in the ISA-TAB-Nano example files.
NanoParticle Ontology (NPO)
Like ISA-TAB, ISA-TAB-Nano provides fields for entering and referencing terms selected from ontologies and standard terminologies. The ontologies are available at BioPortal , which the National Center for Biomedical Ontologies maintains. Though the investigator may use alternative ontology and vocabulary sources, the ability to evaluate and share data require that all parties have access to those being used (they should be available to the investigators). All terms and fields used in this standard utilize the NCI EVS and NanoParticle Ontology elements.
NanoParticle Ontology (NPO) is an ontology that is designed and developed within the framework of the Basic Formal Ontology (BFO) and implemented in the ontology web language (OWL). It is being developed to represent the knowledge underlying the description, preparation, and characterization of nanomaterials. NPO development began with the representation of knowledge underlying the chemical composition, preparation, physiochemical, and functional/biological characterization of nanoparticles that are formulated and tested for applications in cancer diagnostics and therapeutics. The NPO provided the knowledge framework for developing the ISA-TAB-Nano material file format. The NPO provides a subset of the terms and relationships for the description and characterization of nanomaterials in the ISA-TAB-Nano file format. The NPO is being further developed for the following purposes: (1) to provide terms for annotating nanotechnology research data; (2) to provide the knowledge framework required for developing data-sharing models and standards in nanomedicine; (3) to enable semantic integration of data; (4) to enable unambiguous interpretation of the description and characterization of nanomaterials; and (5) to enable knowledge-based searching and comparison of nanomaterial descriptions and characterization results.
ISA-TAB-Nano Extensions to ISA-TAB
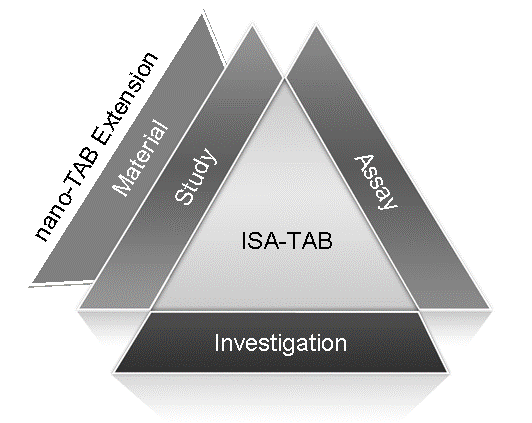
While ISA-TAB-Nano leverages the three primary ISA-TAB files, it extends ISA-TAB by providing specification for a fourth file (called the material file) for representing the composition and characteristics of nanoparticle formulations and small molecules. Raw/derived data files and any other files (for example, image files, protocol documents) specific to each assay have to be shared along with the four primary ISA-TAB-Nano files. ISA-TAB-Nano does not provide any specification for how to format files other than the four primary files: investigation, study, assay and material files. Although ISA-TAB-Nano adopts ISA-TAB field names and their definition in the investigation, study, and assay files, some of the definitions are modified and additional fields are introduced. These modifications and extensions are required to expand the scope of information captured from nanotechnology data sets into the ISA-TAB-Nano files.
Distinction between Biological and Non-biological Samples
In nanotechnology, samples from biological and non-biological sources can be the primary subjects of a study. Therefore, in ISA-TAB-Nano, samples derived from biological sources are called biological specimens or biospecimens (for example, cell line, body fluids, organs, etc.). Whereas, samples derived from non-biological sources are simply called material samples (for example, nanomaterials, nanoparticle formulations, small molecules). For physico-chemical characterizations of nanomaterials, the sample is the nanomaterial. For in-vitro and in-vivo characterizations, the sample is the biological specimen (cell line, animal, and so forth). Hence, in ISA-TAB-Nano, the concept of a sample (as used in ISA-TAB specification) is redefined to include both biological specimens and material samples. The ISA-TAB study file can only be used to record the source and characteristics of biospecimens studied in an assay, and cannot support the representation of materials. Therefore, in ISA-TAB-Nano, the material file describes material samples, while the study file describes biospecimens.
Extensions to ISA-TAB Files
The Table below represents extensions made to each ISA-TAB file in support of ISA-TAB-Nano.
ISA-TAB Extension Field Name | ISA-TAB File | Purpose |
|---|---|---|
Investigation Disease | Investigation | To capture and retrieve investigations associated with specific disease modalities such as cancer |
Investigation Disease Term Accession Number | Investigation | To enable semantic interoperability for disease terms |
Investigation Disease Term Source REF | Investigation | To enable semantic interoperability for disease terms |
Investigation Outcome | Investigation | To enable researchers to review the outcomes of investigations for assessing the utility of the investigation in achieving scientific endpoints |
| MATERIAL | Investigation | To allow for the identification of materials used in the investigation and associated studies |
| Material File Name | Investigation | To identify the name of the Material File, which lists information about the composition and characteristics of the nanomaterials or other small molecules |
| Material Source Name | Investigation | To identify the name of the nanomaterial or small molecule associated with the Material File |
Study Disease | Investigation | To capture and retrieve studies associated with specific disease modalities such as cancer |
Study Disease Term Accession Number | Investigation | To enable semantic interoperability for disease terms |
Study Disease Term Source REF | Investigation | To enable semantic interoperability for disease terms |
Study Outcome | Investigation | To enable researchers to review the outcomes of studies for assessing the utility of the investigation in achieving scientific endpoints |
Study Assay Measurement Name | Investigation | To capture the variables measured in an assay to support cross study analysis |
Study Assay Measurement Name Term Accession Number | Investigation | To enable semantic interoperability for assay measurement names |
Study Assay Measurement Name Term Source REF | Investigation | To enable semantic interoperability for assay measurement names |
Measurement Value | Assay | To record the endpoint value of an assay measurement within the assay file |
All Fields | Material | To describe nanomaterials and small molecules |
ISA-TAB-Nano File Structure
ISA-TAB-Nano uses the three primary files of ISA-TAB-- investigation file, study file, and assay file; and, introduces a fourth file called the material file (Figure 1). Other files such as raw/derived data files, image files, protocol documents, etc., referenced in the ISA-TAB-Nano files have to be shared along with the ISA-TAB-Nano files.
Figure 1. ISA-TAB-Nano File Structure
ISA-TAB specifies that the names of the primary files end with .txt extensions. ISA-TAB-Nano file names may end in either .txt or .xls extensions. The ISA-TAB-Nano files used as examples in this document were prepared in Microsoft Excel spreadsheets, and so their filenames have the .xls extension.
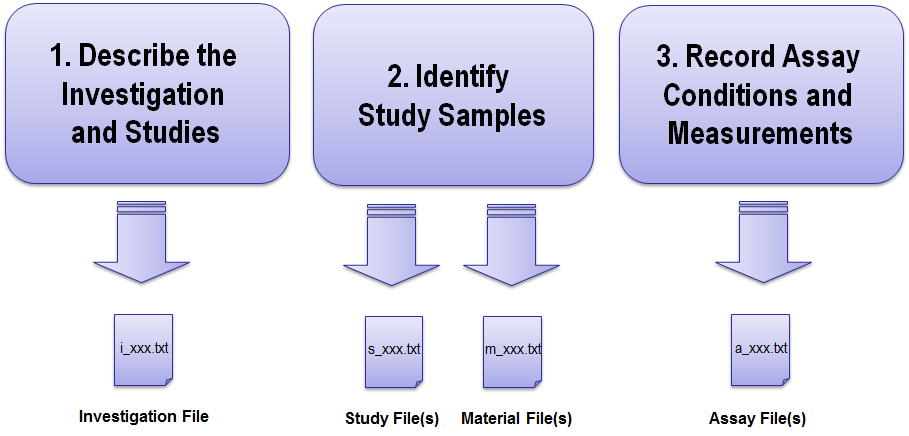
Figure 2 describes the ISA-TAB-Nano file development process.
Typically, the investigation file is developed first and describes the overall investigation, associated studies and assays. The investigation file is a text file with a naming convention of “i_xxx.txt” or “i_xxx.xls,” in which xxx can be any name provided by the investigator. Once the investigation file has been completed, one or more study files (following the convention “s_xxx.txt” or “s_xxx.xls”) can be created. Similarly, one or more material files can be created. The material file describes the nanomaterial (or small molecule) and its components including structural information and follows the naming convention “m_xxx.txt” or “m_xxx.xls”. Assay files (following the convention “a_xxx.txt” or “a_xxx.xls”) are created for all assays performed. Each assay is defined by the endpoint measured and the technique used to measure that endpoint. Data files (raw or derived) specific to each type of assay can be associated to the respective assay files by referencing the names of the data files in the assay files.
Figure 2. ISA-TAB-Nano File Development Process
When sharing primary ISA-TAB-Nano files, other files referenced in these files have to be shared along with the primary files. It is anticipated that content management systems will become available to facilitate the sharing and exchange of files. Until then, these files could be bundled together in a folder and shared as a zip file.
Once the ISA-TAB-Nano files have been created, the files can be validated and submitted into nanotechnology resources that support the ISA-TAB-Nano specification. It is anticipated that validation of the files may occur via a validation service that leverages a modified version of the ISA-TAB validator .
It is also anticipated that nanotechnology resources like caNanoLab (https://cananolab.nci.nih.gov/caNanoLab/ ), the Nanomaterial-Biological Interactions (NBI) knowledgebase and other resources will provide facilities for importing/exporting ISA-TAB-Nano files as the ISA-TAB-Nano specification evolves.
ISA-TAB-Nano File Templates
The following pages provide templates for each ISA-TAB-Nano file:
Definitions of terms in each file are available in the ISA-TAB-Nano Glossary Files.
ISA-TAB-Nano Example Files
The following are example ISA-TAB-Nano files for a nanotechnology investigation involving physico-chemical and in vitro characterization studies.
Investigation Title: Nanotechnology Characterization Laboratory (NCL) Dendrimer-Based MRI Contrast Agent
Investigation Description: The objective of this investigation is to characterize a PAMAM dendrimer with an associated gadolinium chelate MRI contrast agent. The nanomaterials submitted for testing at the NCL were:
- (NCL20) G4 tris (hydroxyl) terminated PAMAM dendrimer
- (NCL21) G4 pyrrolidinone terminated PAMAM dendrimer
- (NCL22) G4.5 COONa terminated PAMAM dendrimer
- (NCL23) G4.5 COONa terminated PAMAM dendrimer-Magnevist® complex
- (NCL25) G4 tris (hydroxyl) terminated PAMAM dendrimer-Magnevist® complex
- (NCL26) G4 pyrrolidinone terminated PAMAM dendrimer-Magnevist® complex
Commercially available Magnevist® (NCL24) was used as a control. The NCL example files encompass three categories: physicochemical characterization; immunotoxicology; in vitro toxicology.
Example Files
- Investigation File: NCL Dendrimer-Based MRI Contrast Agent
- Study Files: Size by Dynamic Light Scattering (DLS) | Cytotoxicity Study
- Material Files: NCL-20 | NCL-21 | NCL-22 | NCL-23 | NCL-24 | NCL-25 | NCL-26
- Assay Files: Size by DLS Assay | Lactate Dehydrogenase (LDH) Assay | MTT Assay
- Report: Dentrimer-Based MRI Image Contrast Agent
Help Downloading Files
For help accessing PDF, audio, video, and compressed files on this wiki, go to Help Downloading Files.