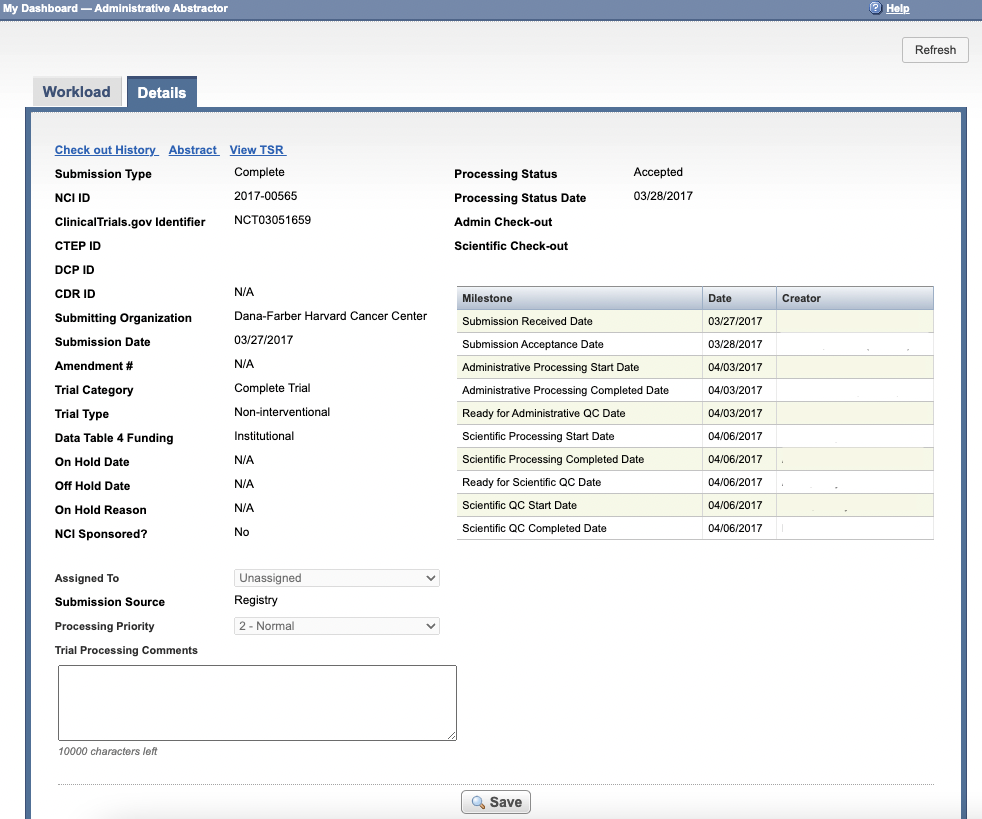
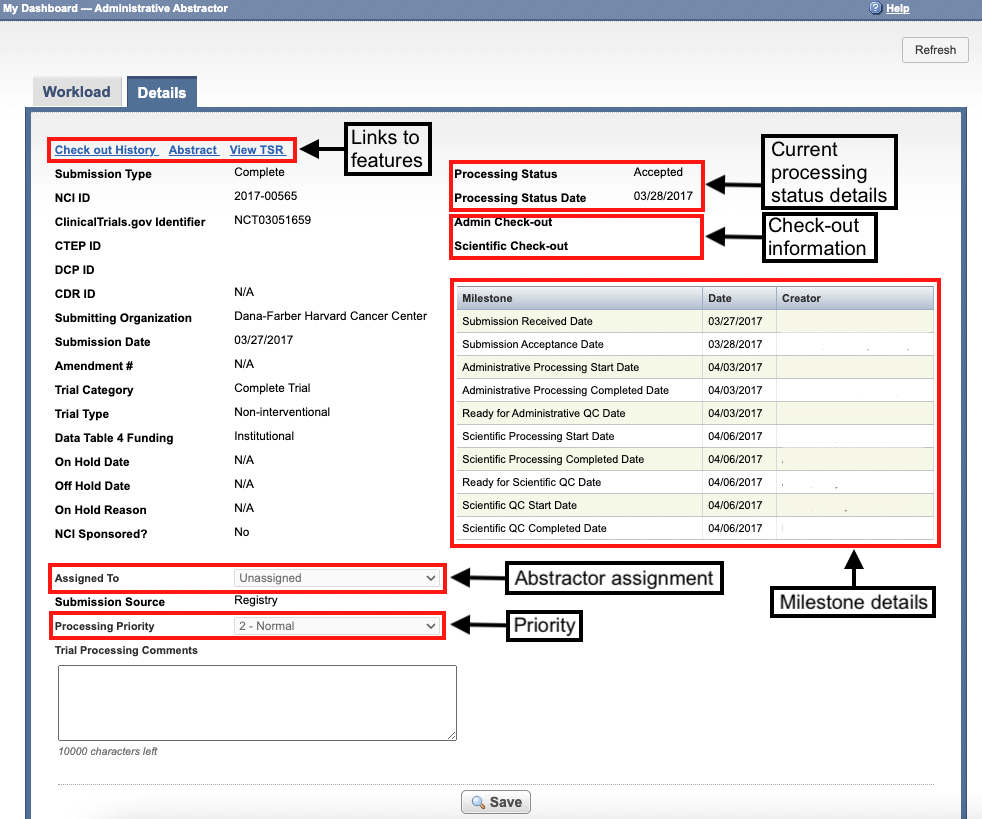
The abstraction dashboard's Details page displays information about the trial you select from the abstraction dashboard's Workload page.
The Details page includes the following trial details:
| Field | Description |
|---|---|
| Submission Type | The submission type:
For information, refer to Processing Trial History Information (original versus amendment) or Abstracting NCI-Specific Information (Complete versus Abbreviated). |
| NCI ID | The unique ID assigned to the trial by the CTRP. NCI Trial Identifiers The prefix "NCI" is implicit in the NCI trial identifiers. |
| ClinicalTrials.gov Identifier | The unique ID assigned to the trial by the National Clinical Trial program (ClinicalTrials.gov) for trials that have been submitted to ClinicalTrials.gov Protocol Registration System (PRS) previously. This ClinicalTrials.gov ID appears as "NCT" followed by 8 numeric characters (such as NCT12345678). For details, refer to Abstracting Trial Descriptions, Titles, and Identifiers. |
| CTEP ID | The unique ID assigned to the trial submitted by CTEP (Cancer Therapy Evaluation Program). For details, refer to Abstracting Trial Descriptions, Titles, and Identifiers. |
| DCP ID | The unique ID assigned to the trial submitted by DCP (Division of Cancer Prevention). For details, refer to Abstracting Trial Descriptions, Titles, and Identifiers. |
| CDR ID (PDQ ID) | The number assigned to trials in the Clinical Data Repository. For details, refer to Abstracting Interventions. |
| Submitting Organization | The organization associated with the CTRP user who submitted the trial to CTRP. |
| Submission Date | The date on which the CTRP user submitted the trial. |
| Amendment # | If the trial submitter specified a value as the amendment number while amending the trial, this field displays that value. (This value may be a text string, whereas the submission number is numeric.) For instructions on revising this value, refer to Editing Trial Submission History. |
| Trial Category | The category of the trial, as determined by the submission of a full protocol (Complete) or a ClinicalTrials.gov import (Abbreviated). For information, refer to CTRP Trial Categories, Study Sources and Abstracting NCI-Specific Information. |
| Trial Type | The primary investigative techniques used in the protocol (interventional or non-interventional). The non-interventional category includes observational and ancillary/correlative studies. For information, refer to Trial Types and Subtypes, Abstracting Interventional Trial Design, and Abstracting Non-Interventional Trial Design. |
| Data Table 4 Funding | The type of Data Table 4 funding sponsorship (National, Externally Peer-Reviewed, Institutional, or Industrial). For information, refer to CTRP Trial Categories, Study Sources and Abstracting NCI-Specific Information. |
| On Hold Date | The date on which the abstractor put the trial on hold, if applicable. For details, refer to Putting Trial Processing On Hold. |
| Off Hold Date | The date on which the abstractor took the trial off hold, if applicable. For details, refer to Putting Trial Processing On Hold. |
| On Hold Reason | The reason why the abstractor put the trial on hold, if applicable. For details, refer to Putting Trial Processing On Hold. |
| NCI Sponsored? | An indication whether National Cancer Institute (NCI) sponsored the trial. For details, refer to Abstracting Sponsors and Responsible Parties. |
| Assigned To | Displays the name of the abstractor assigned to the trial by a Super Abstractor. Only users with the Super Abstractor role can assign a trial to another PA user. For information, refer to Assigning Trials to Abstractors. |
| Submission Source | The means by which the trial was submitted to the CTRP:
|
| Processing Priority | The importance assigned to the trial by a Super Abstractor: Only users with the Super Abstractor role can assign a priority to a trial. For information, refer to Assigning Trial Priorities.
|
| Trial Processing Comments | Any comments that an abstractor has recorded for this trial. This field also allows you to enter new comments about the trial. An abstractor can also add comments on the Trial Identification page. For information, refer to Viewing Trial Identification Details. |
| Processing Status | The current status of the trial in the CTRP trial processing work flow. For information, refer to Trial Processing Statuses and Abstracting Trial Statuses. |
| Processing Status Date | The date on which the current processing status was recorded. |
| Admin Check-out | The Administrative Abstractor who has the trial checked out currently (if applicable). For details, refer to Checking In and Checking Out Trials. |
| Scientific Check-out | The Scientific Abstractor who has the trial checked out currently (if applicable). For details, refer to Checking In and Checking Out Trials. |
| Milestone (table) | A list of the milestones recorded to date. For details, refer to Processing Trial Milestones. |
How to Use the Details Page
| To do this... | Do this |
| Check the trial in or out | Click the Admin Check In/Out link. The trial is checked out to you, preventing other users from abstracting the same trial at the same time. This link is not available when the trial is currently checked out by another Administrative Abstractor. Current check-out information is displayed on the Details page. If a Super Abstractor assigned the trial to you, it will already be checked out to you when you log in. You can check it back in when you have finished processing it. See Checking In and Checking Out Trials. |
| View check-out history | Click the Check-Out History link. The Trial Check-Out History page displays records of each check-out/check-in event. For details, see Viewing Check-Out History Records . |
| Accept the trial | Click the Validate link. This link is available only for trials that have been submitted but not accepted yet. |
| Abstract the trial | Click the Abstract link. |
| View the TSR | Click the View TSR link. |
| Enter comments | Enter text in the Trial Processing Comments text box, then click Save. The comment field is limited to 10,000 characters. |
In the figure above, a Super Abstractor has not assigned the trial to a particular abstractor for processing, and has kept the default processing priority (2 - Normal).