|
Page History
...
- On the toolbar, click Search > Clinical Trials.
The Search Trials page appears. - Click Search > My Trials.
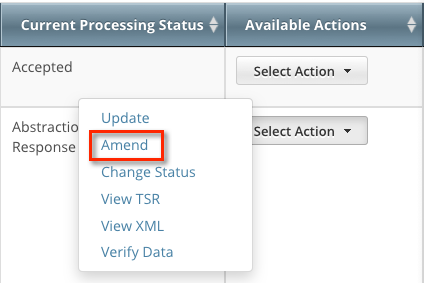
The Search Results page displays the results of your search and actions available (if any) for each record.
In the Available Actions column, click Select action > Amend. The Amendment Trial page displays the data currently registered with the CTRP.
HTML Comment hidden true Screenshot TBD.
Make changes to the fields as necessary. The instructions are the same for trial registration and trial updateamendment, with some exceptions:
In the Amendment Details section, select or enter the appropriate information in the drop-down lists and text fields. The following table describes the fields. An asterisk (*) indicates a required field.
Field Label Description/Instructions Amendment Number
Enter an appropriate number.
Amendment Date* Select or enter an appropriate date. Select or enter the appropriate information in the remaining text fields and drop-down lists, following the instructions provided in Registering New Trials .
Info Info You are required to provide information for all fields marked with an asterisk (*).
Note You can select a different disease code only if the trial has not accrued any subjects to date.
- Review the amendment. See Reviewing and Submitting Trial Amendments .
- Submit the amended trial to the CTRP.
The system sends you an email notification — with the details of what has changed — whenever you amend accepted trials.
...