Module Status Table
** Modules are alphabetized
** Content that is marked Final is ready for use. Content that is marked Under Review is candidate status and is available to use but please note that this content could change in the final release.
CRF Modules | Round # | Status | Status Description |
|---|---|---|---|
2 | Final | Total of 31 CDEs built in two CRF standard modules – one to use with CTCAE v3.0 reporting requirements, and one for CTCAE v4.0 reporting requirements. AE modules include Serious Adverse Event (SAE) CDEs. | |
Base Interventions | 3 | Not Needed - Removed | The intent of the Base Interventions module was to group all commonly-used CDEs need for primarily intervention studies. In one module CDEs for start date, stop date, dose, Unit of measure, dose, etc., were grouped. Reviewers did not feel this was the best way to categorize the elements. So the pertinent elements in the Base Interventions module were instead added to each intervention/agent module. The Base Interventions module is retired, and not part of the final Standard CRF set. |
3 | Final | Total of 13 CDEs built in one CRF standard module. | |
4 | Final | Total of 6 CDEs built in one CRF standard module. | |
| CT Image Acquisition | 5 | Under Review | |
| CT Imaging Agent | 5 | Under Review | |
1 | Final | Total of 15 CDEs built in one CRF standard module. | |
4 | Final | Total of 8 CDEs built in one CRF standard module. | |
4 | Final | Total of 6 CDEs built in one CRF standard module. | |
| Diagnosis | 5 | Under Review | |
| Diagnosis Gross Pathology | 5 | Under Review | |
| Diagnosis Metastasis | 5 | Under Review | |
| Diagnosis Microscopic Pathology | 5 | Under Review | |
Drug Compliance | 3 | Not Needed - Removed | Review demonstrated that 5 of the 8 CDEs in this module are repeated in the Study Administration CRF module. So the remaining 3 CDEs were merged with the Study Administration CRF standard module, and the Drug Compliance module removed as a separate CRF module. |
4 | Final | Total of 8 CDEs built in one CRF standard module. | |
3 | Final | Total of 8 CDEs built in one CRF standard module. | |
2 | Final | Total of 5 CDEs built in one CRF standard module. | |
4 | Final | Total of 10 CDEs built in one CRF standard module. | |
| Follow-Up | 5 | Under Review | |
3 | Final | Total of 5 CDEs built in one CRF standard module. | |
3 | Final | Total of 11 CDEs built in one CRF standard module. | |
| Image Administration | 5 | Under Review | |
4 | Final | Total of 7 CDEs built in one CRF standard module. | |
3 | Final | Total of 14 CDEs built in one CRF standard module. | |
| Lost to Follow-up | 5 | Under Review | |
2 | Final | Total of 7 CDEs built in one CRF standard module. | |
3 | Not Needed - Removed | The outcome module was created in round 2. At the time of delivery the limitations in the content were recognized, and we had a plan to expand the content at some point and also look mainly at response results in nonsolid tumors, like the leukemias and lymphomas. So round 5 was envisioned as expanding two main areas in round 2 – 1) responses had to be expanded beyond the solid tumors, and 2) survival had to be expanded. Therefore, Outcomes in round 2 have now been replaced by content in round 5. | |
4 | Final | Total of 3 CDEs built in one CRF standard module. | |
4 | Final | Total of 3 CDEs built in one CRF standard module. | |
2 | Final | Total of 10 CDEs built in one CRF standard module. | |
4 | Final | Draft New CRF standard module undergoing community review and vetting. | |
4 | Final | Total of 2 CDEs built in one CRF standard module. | |
4 | Final | Draft New CRF standard module undergoing community review and vetting. | |
4 | Final | Total of 7 CDEs built in one CRF standard module. | |
| Progression | 5 | Under Review | |
2 | Final | Total of 6 CDEs built in one CRF standard module. | |
3 | Final | Total of 14 CDEs built in one CRF standard module. | |
2 | Final | Total of 8 CDEs built in one CRF standard module. The use of a Protocol Deviation module is NOT recommended. Deviation data should be recorded in the appropriate form (agent administration, AE, etc.). However, if a sponsor mandates the use of an additional Protocol Deviation module, the identified variables should be used. | |
4 | Final | Total of 30 CDEs built in one CRF standard module. | |
| RECIST | 5 | Under Review | |
2 | Final | Total of 19 CDEs built in one CRF standard module. | |
| Response | 5 | Under Review | |
4 | Final | Total of 4 CDEs built in one CRF standard module. | |
3 | Final | Total of 21 separate CRF modules for major disease groups, including Leukemia, Lymphoma, and Solid tumors. There is no generic stage CRF module content. All CRF modules begin with ‘Staging’ and then have the specific diagnostic group. CDEs in these modules are largely but not exclusively based on AJCC edition 7.0 collaborative staging criteria. | |
3 | Final | Total of 19 CDEs built in one CRF standard module. | |
4 | Final | Total of 31 CDEs built in one CRF standard module. | |
| Survival | 5 | Under Review | |
4 | Final | Total of 24 CDEs built in one CRF standard module. |
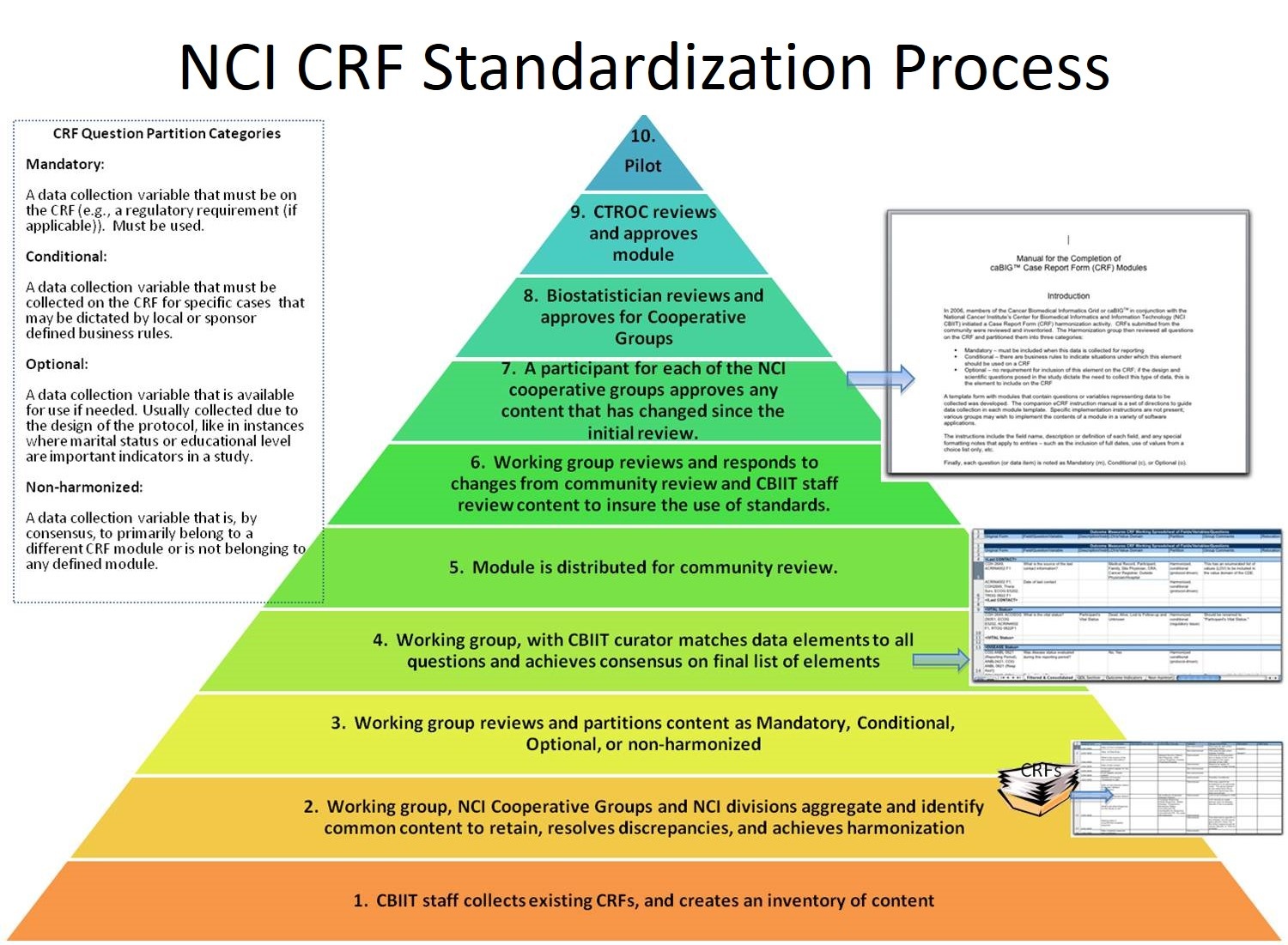
Harmonization/Standardization Process
The CRF Harmonization and Standardization initiative has undertaken the task of harmonizing and standardizing case report forms for cancer clinical trials by first dissecting the CRF into modules and tackling smaller sections of the CRF 5 areas at a time. The general process includes collecting an inventory of forms related to the information generally captured in that module, for example, On-Study Forms are collected to gather Agent information. The group then goes through all the forms and identifies those fields that are relevant to that module (area), partitions the fields as either Mandatory, Conditional or Optional, and then identifies appropriate Common Data Elements (CDEs) or creates new CDEs to accurately capture the metadata for the harmonized fields. Once the group identifies all the fields and CDEs to be collected in that module, these are sent to the Clinical Trials Community for review and comment, the comments are addressed, and the final list of fields are presented to the Clinical & Translational Research Operating Committee (CTROC) for final approval. After CTROC approval, the CDEs are brought to the Vocabularies & Common Data Elements (VCDE) Workspace for review as caBIG Standards. Once the CDEs are made standard, the module is officially available for use on cancer clinical trials.
In October of 2009, the need for an Expanded Committee Review was identified. A change in the community review process was instituted in the Spring of 2010. The revised process is as follows:
Status of CRF Activities
The first Round of the CRF Harmonization and Standardization Project addressed a single module, Demography. This work began in June 2007, and the module has gone through the the entire process and has been approved by CTROC, and the CDEs have been made standard. The module is undergoing implementation/deployment for use in cancer clinical trials.
Round 2 included Adverse Events, Baseline Assessment, Participant Identification, Registration & Enrollment, and Protocol Deviations. Seven modules were completed from those 5 areas, which are Adverse Events, Medical History, Physical Exam, Participant Identification, Registration, Enrollment, and Protocol Deviations. These seven modules have been vetted by the Community and approved by CTROC. In the spring of 2010 the seven modules entered expanded community review. Community review completed three rounds of evaluation through 2010 and in March of 2011 was forwarded to NCI leadership for finalization.
Round 3 modules include Agents (looking at both standardizing vocabulary and the variables to be collected), Laboratory Tests/Results, Outcome Measures, and Staging/Extent of Disease. These modules have been reviewed by the Community, presented to CTROC, and are undergoing final review by specific groups in the cancer clinical trials community. It is anticipated that this set of content will be finalized in April of 2011.
The Round 4 modules: Header Information, Imaging/Radiology, Non-agent Study Interventions, Diagnosis/Pathology, Vital Signs, Eligibility Criteria, and Off Treatment/Off Study have completed the first round of harmonization in small workgroups. They will enter expanded community review upon the completion of Round 3 content, sometime in the spring of 2011.
Round 5 modules have not been identified at this point, but most likely will include Imaging variables, specifically Recist criteria. Additional content will be identified following a gap analysis once Round 4 work is complete.
Metrics
The CRF Harmonization & Standardization project was initiated to provide a harmonized and standardized set of variables to be collected for oncology clinical trials that could be implemented to facilitate data entry, and study aggregation, comparison and analysis. The CRF collection process for Rounds 1-3 only collected Case Report Forms specific for that module. However, prior to Round 4, the Workgroup Leads recommended collecting CRFs for all areas and doing a single CRF inventory for all remaining modules, current and future Rounds.
The general metrics from the Round 4 CRF Inventory include:
17 Contributing Organizations
Contributing Organizations: CCR, DCP, University of Pennsylvania, CDASH, NCCTG, CTMS Theradex, CIP (FIMSO), UCSD, CALGB, COH, ACOSOG, Duke, ACRIN, ECOG, University of Michigan, Baylor College of Medicine, and RTOG
237 Case Report Forms
7697 variables (total)
More metrics on the CRFs, variables and modules will be posted on this page and the Project Metrics page.