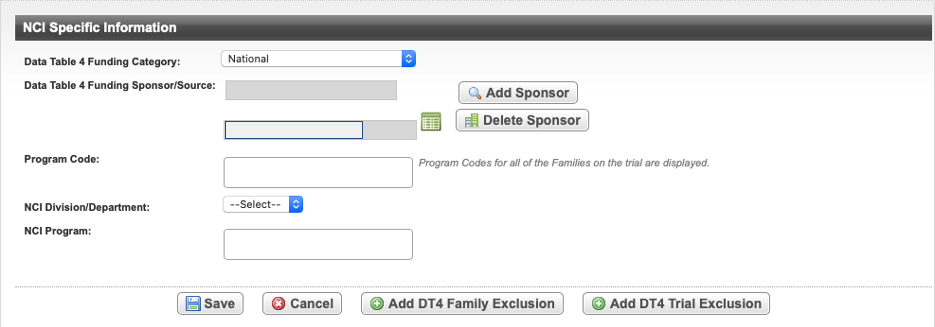
Abstracting NCI-Specific Information
Search for the trial of interest. For instructions, refer to Searching for Trials in PA.
In the search results, Select the NCI Trial Identifier link for that trial. The Trial Identification page opens.
On the Trial Identification page, check out the trial. For instructions, refer to Checking In and Checking Out Trials. (This checkout step is optional for Super Abstractors.)
On the Administrative Data menu, click NCI Specific Information. The NCI Specific Information page opens.
The following table describes the fields on the NCI Specific Information page.
Field Label
Description/Instructions
Data Table 4 Funding Category
Select a trial type based on the role/responsibility/participation in the study. For information, refer to CTRP Trial Categories, Study Sources and https://cancercenters.cancer.gov/GrantsFunding/eData#dt4.
Data Table 4 Funding Sponsor/Source
To add a sponsor, select Add Sponsor and search for the name of the external sponsor or funding source as defined by the Data Table 4 report. (See Searching for Organizations.)
A trial can have multiple sponsors. CTRP ensures don't duplicate sponsor cannot be entered.
To delete an existing sponsor, select Delete Sponsor.
Refer to https://cancercenters.cancer.gov/GrantsFunding/eData#dt4 for further information about specific Funding Sponsors.
Industrial? For Abbreviated trials, indicate whether the trial is an Industrial trial, or other category, according to the matrix in Industrial Values.
Program Code
Only available if the Lead Organization for the trial belongs to an organization family.
The Program Code field lists all program codes available for the organization family of the lead organization. Select one or more program codes. The program codes are generally entered by the trial submitter.
To view or modify a different family's program codes, refer to the Registration Site Administration chapter of the Registration User's Guide.
Send Trial Information to ClinicalTrials.gov? For Complete, NCI Sponsored trials, select one of the following to indicate whether to include this trial in the nightly batch upload to ClinicalTrials.gov:
- Yes. Trial will be included in the nightly batch to ClinicalTrials.gov.
- No. Trial will not be included in the nightly batch to ClinicalTrials.gov. CTRP will still display this field and allow this value to be updated to Yes.
Selecting Yes: Once PRS indicates to CTRP that the trial in the nightly batch processed successfully, CTRP will hide this field for the trial.
For further information about this field, see Conditions for Sending Trial Information to ClinicalTrials.gov.
Comments Enter a comment about your selection. NCI Division/Department Select the division or department that is managing the trial.
# Value
Definition
3 CCR
Center for Cancer Research
6 CIP
Cancer Imaging Program
1 CTEP
Cancer Therapy Evaluation Program
4 DCCPS
Division of Cancer Control and Population Sciences
7 DCEG
Division of Cancer Epidemiology and Genetics
2 DCP
Division of Cancer Prevention
NCI Program Select one or more relevant programs.
# Value
Definition
1 BIQSFP Trials maintained by the Biomarker, Imaging, and Quality of Life Studies Funding Program. 6 ETCTN Trials maintained by the Experimental Therapeutics Clinical Trials Network. 4 NCORP Trials maintained by the Community Oncology Research Program. 5 NCTN Trials maintained by the National Clinical Trials Network. 2 SPORE Trials maintained by the Specialized Programs of Research Excellence. 3 Steering Committee Trials maintained by leading cancer experts, community oncologists, biostatisticians, translational scientists, and advocates as well as NCI senior investigators. - Optionally, to view the details of the Data Table 4 Funding Sponsor/Source (if already recorded), click the Details icon ().
Displaying Organization and Person Details
You can display the details of any organization or person, including their CTEP and PO IDs, that appears on abstraction pages by clicking the Details icon () located next to an organization or person name field.
- To save the details you have abstracted, click Save.
For more information, refer to the following pages: