You can search for–and abstract or edit–any registered trial that has been submitted successfully to the Clinical Trials Reporting Program (CTRP). Search criteria are available to locate a particular trial to work on, or you can list all registered trials that are available for validation and abstraction. You also can limit your search to the trials you have checked out previously.
Super Abstractors can use the abstraction dashboard feature to search for trials as well. See Tracking and Managing Trial Records.
How to Search for Trials
If the Trial Search page is not already displayed, on the Protocol Abstraction main menu on the left side of the page, click Trial Search. The Trial Search page appears.
Type or select any of the search criteria listed in the following table as appropriate.
You can select multiple values for Principal Investigator, Trial Phase, Processing Status, Search by Submission Type, Search by Submission Method, Lead Organization, Primary Purpose, Current Trial Status, and Milestone.
Search Criteria
Description/Instructions
Official Title
Type any portion of the name of the clinical trial as it appears in the protocol document, using wildcards as appropriate.
Avoid copying and pasting the entire title into the search field
Use keywords and wildcards rather than phrases or the entire title. Doing so minimizes the potential for excluding from the search results any titles with misspellings or slightly different phrasing.
Identifier Type
Select the type of identifier you want to search for from the drop-down list. For identifier type descriptions, see the list of identifiers in Abstracting General Trial Details.
Searching by Identifiers
You do not have to select an Identifier Type when searching by Trial Identifier
Identifier
Type all or part of the numeric or alphanumeric identifier for the type of identifier you selected. Examples:
- NCI-2000-00015
- ECOG-1234
Inter-Group trials use the lead Group’s trial number.
Principal Investigator
Select the name of the individual who is responsible for all aspects of the conduct of the study from the drop-down list.
Search tip
To narrow the list of investigators displayed, click the Principal Investigator field and type the initial letter of the last name. You can also use the Up and Down arrow keys on your keyboard to scroll through the list.Trial Phase
Select the phase of the investigation, as defined by the US FDA for trials involving investigational new drugs, from the drop-down list. See Abstracting Interventional Trial Design.
Processing Status
Select the processing status from the drop-down list. For processing status definitions, see Trial Processing Statuses.
Search by On-Hold Status
Select the On-Hold Status from the drop-down list. Valid values are as follows:
- All. Search for trials without consideration of On Hold status.
- On Hold. Limits your search to trials that are currently on hold.
- Not On Hold. Limits your search to trials that are currently not on hold.
Search by Submission Type
Select the submission type from the drop-down list. Valid values are as follows:
- All. Search for original and amended submissions
- Original. Search for original submissions only (indicated by “O” in the results list)
- Update. Search for updated submissions only (indicated by “U” in the results list)
- Amendment. Search for amended submissions only (indicated by “A” in the results list)
Trial Type
Select the primary investigative techniques used in the protocol from the drop-down list. Valid values are as follows:
- All. Search for all trial types
- Interventional. Search for Interventional trials only
- Non-Interventional. Search for Non-interventional trials only
For information, refer to Trial Types and Subtypes.
Trial Sub-type If you search for Non-Interventional trials only, you can limit your search to a trial sub-type. Valid values are as follows:
- Observational. Search for Observational trials only (primary strategy for subject identification and follow-up; epidemiologic, outcome, or other)
- Ancillary-Correlative. Search for Ancillary-Correlative trials only (associated with a clinical trial and other biological studies using clinical specimens that can be linked to individual)
Search by Submission Method
Select the means by which the trial was submitted to the CTRP from the drop-down list. Valid values are as follows: - Batch. Search for trials submitted via the NCI CTRP Registration Batch Upload site
- Registration. Search for trials submitted via Registration
- ClinicalTrials.gov. Search for trials imported from ClinicalTrials.gov
- PDQ. Search for trials submitted via the NCI Physician Data Query database
- Grid Services. Search for trials submitted via the NCI CTRP web services
- Other. Search for trials submitted via any method other than those listed above
Lead Organization
- Select the principal administrative organization responsible for the research protocol from the drop-down list.
Tip
To narrow the list of organizations displayed, click the Lead Organization field and type the initial letter of the organization’s name. You can also use the Up and Down arrow keys on your keyboard to scroll through the list.Primary Purpose
Select the primary reason for the trial from the drop-down list. For primary purpose definitions, refer to Primary Purpose Value Definitions.
Current Trial Status
Select the current stage or state of a clinical trial or study relative to other stages and its ability to enroll participants/patients from the drop-down list. For trial status definitions, see Trial status definitions.
Milestone
Select the current milestone from the drop-down list. For individual milestone definitions, see Processing Trial Milestones. When appropriate, both Administrative and Scientific milestones and their dates are included for each trial in the search results list.
Trials I Have Checked Out
Select this check box if you want to limit your search to trials that you have checked out to validate, abstract, or edit.
Search By Trial Category
Select the trial category from the drop-down list. Valid values are as follows:
- All. Returns all trials, including Abbreviated and Complete trials
- Abbreviated. Returns Abbreviated (Industrial/Other) trials only. Design and implementation of the study is controlled by the pharmaceutical company or by another organization.
- Complete. Returns Complete trials only
- National - National Clinical Trials Network (NCTN) and other NIH-supported National Trial Networks.
- Externally Peer-Reviewed - R01s, SPORES, U01s, U10s, P01s, CTEP, or any other clinical research study mechanism supported by the NIH or an approved peer-reviewed funding organization.
- Institutional - In-house clinical research studies authored or co-authored by Cancer Center investigators and undergoing scientific peer-review solely by the Protocol Review and Monitoring System of the Cancer Center. The Cancer Center investigator has primary responsibility for conceptualizing, designing and implementing the clinical research study and reporting results. It is acceptable for industry and other entities to provide support (e.g., drug, device, other funding) but the trial should clearly be the intellectual product of the center investigator.
This category may also include:- Institutional studies authored and implemented by investigators at another Center,
- Multi-site institutional studies authored and implemented by investigators at your Center.
Search CTEP, DCP, or All Trials
Select the sponsor organization from the drop-down list. Valid values are as follows:
All Trials. Returns all trials, including CTEP and DCP trials
CTEP and DCP PIO. Returns either CTEP or DCP PIO trials
- CTEP PIO Trials Only. Returns CTEP PIO trials only
- DCP PIO Trials Only. Returns DCP PIO trials only
DCP is an abbreviation of Division of Cancer Prevention. CTEP is an abbreviation of Cancer Therapy Evaluation Program. PIO is an abbreviation of Protocol and Information Office.
Click Search, or press the Enter key on your keyboard.
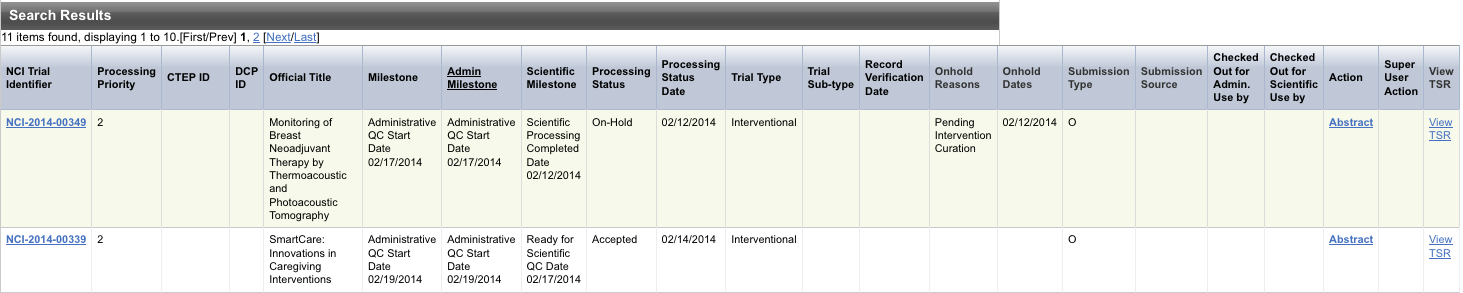
The results of your query are listed in the Search Results section.
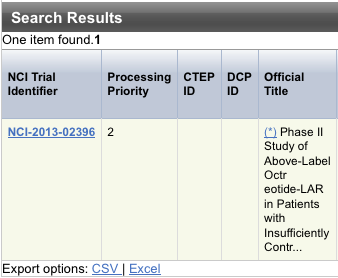
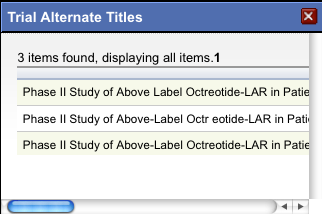
The system displays an asterisk (*) in the Official Title column for trials that have alternate titles.
To view the alternate titles, click the asterisk.
Alternate titles are listed in the Trial Alternate Titles window.
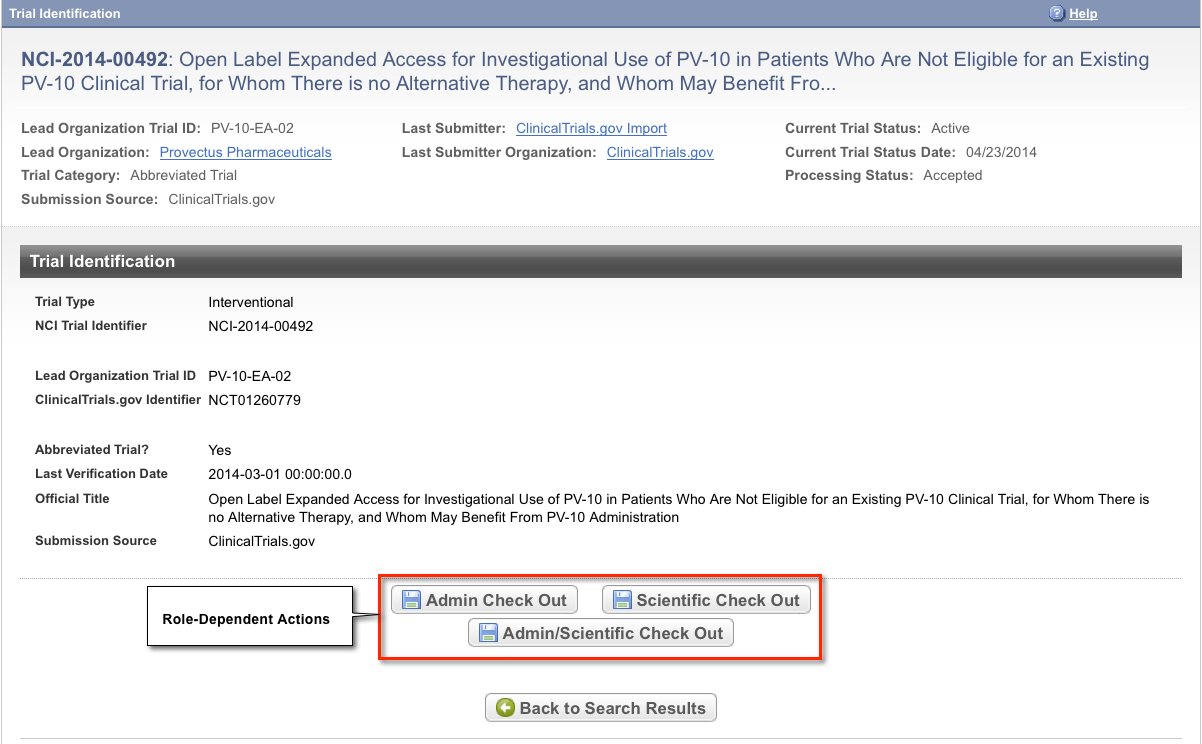
To view details for a trial, click the NCI Trial Identifier link for the trial of interest, or click any link in the Action column.
The Trial Identification page displays trial identifiers and other details. It also displays one or more options to check out the trial for processing. For example, if you logged in to the application as a Super Abstractor, you can check the trial out for both administrative and scientific processing, as in the figure below. However, if you logged in as an Administrative Abstractor, your only option is to check in/out the trial for administrative processing.
Returning to the Search Results page
To return to your most recent search results, click Back to Search Results on the Trial Identification page.- To view a Trial Summary Report (TSR), click the View TSR link for the trial of interest, and open the file.
- To export the search result records, at the upper or lower right corner of the search results table, click one of the following:
- Click CSV to create a generic comma-separated value file.
- Click Excel to create a Microsoft Excel spreadsheet.