This page provides an overview of the changes in CTRP. For any questions or issues regarding these feature enhancements, please contact the CTRO (NCICTRO@mail.nih.gov).
August 2021
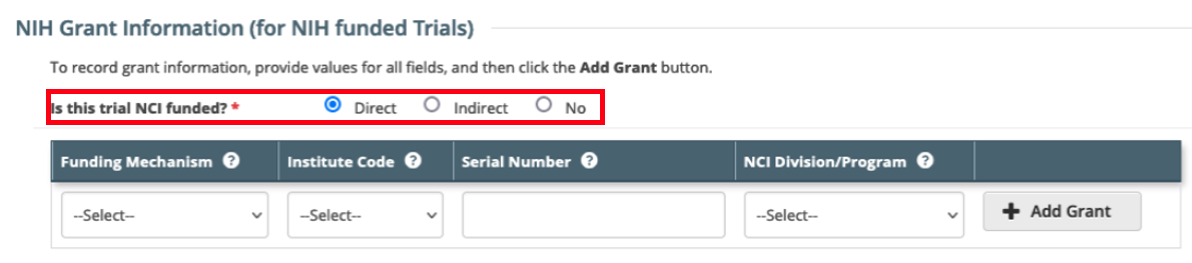
Update to the NIH Grant Information section in the CTRP Registration application. The field 'Is this trial funded by an NCI Grant?' with values of Yes/No has been updated to 'Is this trial NCI funded?' with values of Direct/Indirect/No. This field is available through the web application when registering and amending trials through the CTRP Registration user interface. This update does not have any impact on CTRP REST Services or CTRP REST Service users.
Definitions:
Direct - Trials conducted under any type of contract, grant, or cooperative agreement supported by the NCI (e.g., R01, N01, SPORE, P01, U01, U10) including all National trials (e.g., NCTN, ETCTN, NCORP) and all Intramural trials conducted by the NCI Center for Cancer Research (CCR).
Indirect - All trials conducted at an NCI-Designated Cancer Center (with P30 center core grant), including all industrial trials – without direct NCI funding.
No - NCI has not provided any Direct or Indirect funding for the trial.
For additional information about recording NIH Grant Information in the CTRP Registration application, please visit the following CTRP User Guide page:
July 2021
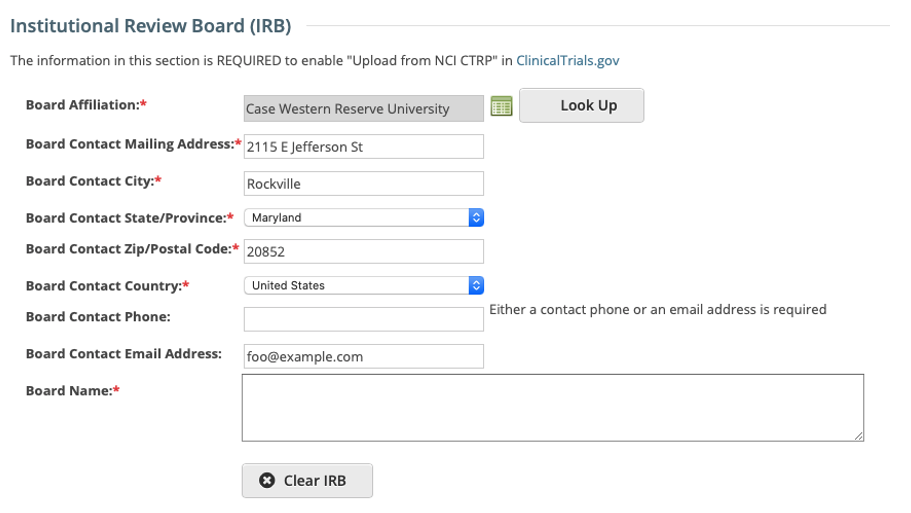
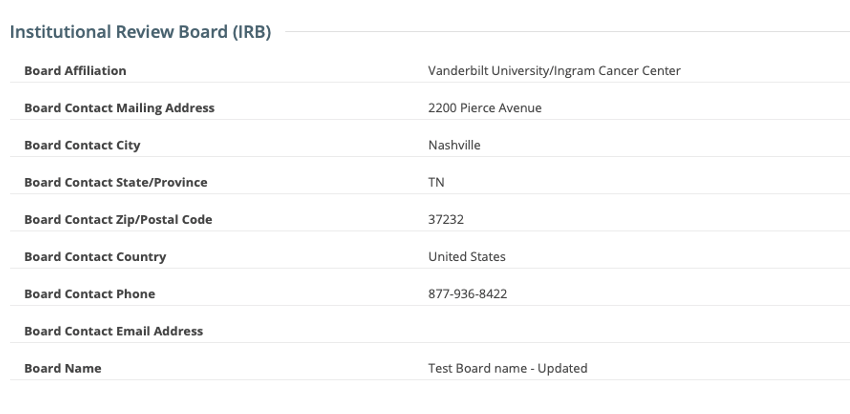
IRB fields are now exposed in the CTRP Registration application. IRB information can be added/modified when registering, updating/verifying and amending non-industrial trials. IRB fields are read-only when viewing trials on the Trial Details Page.
Registering, Amending or Updating/Verifying a non-industrial trial: IRB fields are modifiable
Viewing a trial on the Trial Search page: IRB fields are read-only
For additional information about the IRB fields in the CTRP Registration application, please visit these CTRP User Guide pages:
Recording Institutional Review Board (IRB) Information
Updating Institutional Review Board (IRB) Information in Complete Trials
June 2021
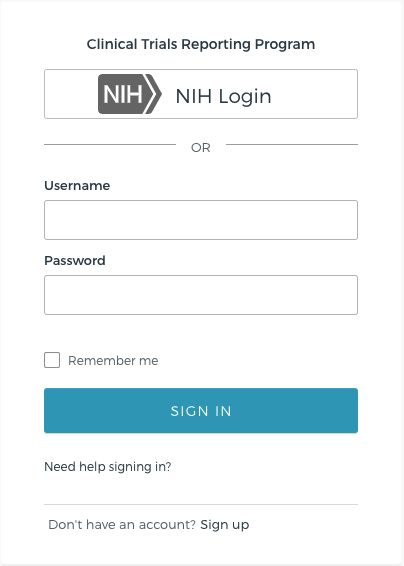
Transition to the NCIs multi-factor authentication system, Okta: Your CTRP username is now your institutional email address.
Migration of CTRP user accounts to Okta. Okta multi-factor authentication is used to provide additional security beyond just entering a username and password. Each Okta account requires the configuration of at least one of the following methods:
- Okta Verify: Use a 'Push Notification' sent to the Okta app on the users mobile device.
- SMS Authentication: Enter a single-use code sent to the users mobile device.
- Voice Call Authentication - Phone call authentication by following voice instructions.
New CTRP login page
For additional information about creating and setting up an Okta account, please visit these CTRP User Guide pages:
March 2021
Updates to the Data Table 4 (DT4) Anatomic Site values displayed on the CTRP-generated DT4 report:
Previous DT4 Anatomic Site Value | Updated DT4 Anatomic Site Value |
Leukemia, not otherwise specified | Leukemia, other |
Breast – Female | Breast |
Breast – Male | Breast |
Hodgkin's Lymphoma | Hodgkin Lymphoma |
Non-Hodgkin's Lymphoma | Non-Hodgkin Lymphoma |
Soft Tissue / Sarcoma | Soft Tissue |
Note: For trials with numerous “Primary Sites” (DT4 Anatomic Sites), the value “Multiple” for the DT4 Anatomic Site Code will be used and displayed on the CTRP-generated DT4 report. All existing trials in CTRP have been updated to reflect this change as well.
For a complete list of DT4 anatomic site values displayed on the CTRP-generated DT4 report, please visit this CTRP User Guide page:
Data Table 4 Anatomic Site Values
Removal of the following fields from the Trial Summary Report (TSR):
- Keywords
- Program Code in the Data Table 4 Section
- Target Accrual in the Participating Sites Section
- Reporting Data Set Method
September 2020
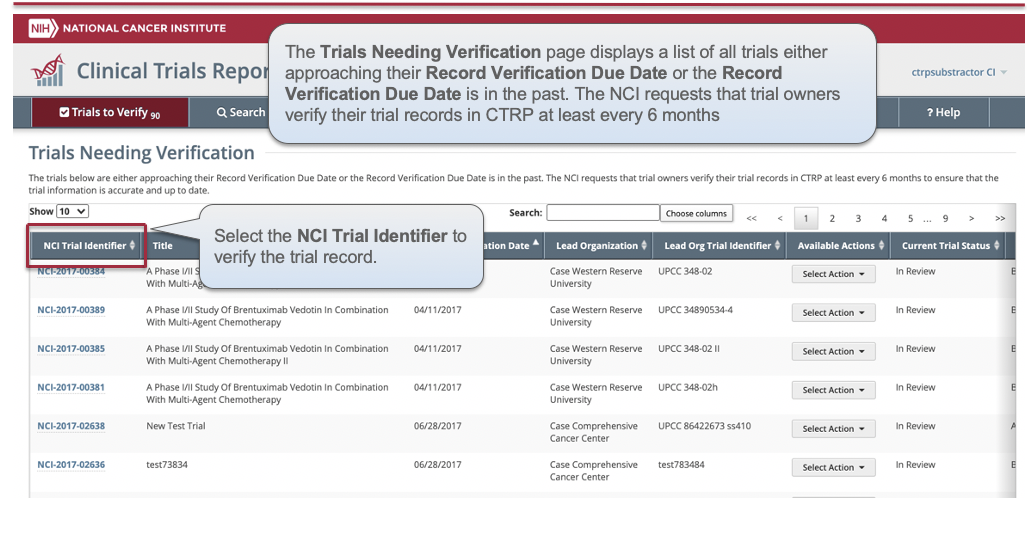
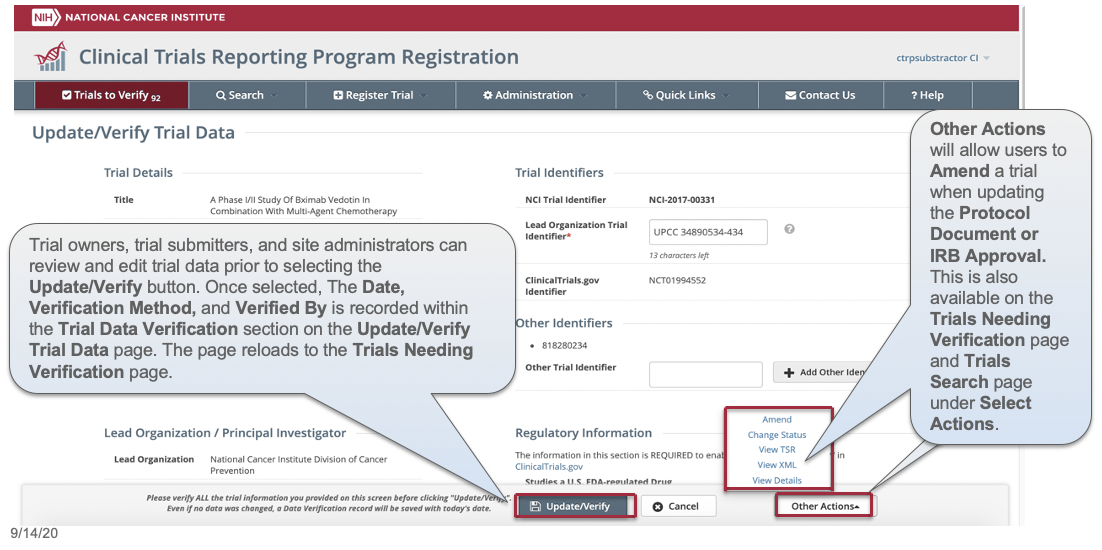
CTRP Trial Record Verification: Improved the user experience for CTRP trial owners, trial submitters and site administrators with Verifying, Updating and/or Amending their trial records. Key highlights from this enhancement include:
- New page highlighting all Trials Needing Verification
- Ability to view all trial details on the Verify screen
- Simplified workflow allowing users to Verify, Update or Amend trials within same form
- New monthly email notification for alerting trial owners, trial submitters,
and site administrators of Trials Needing Verification
For additional information on the CTRP Trial Record Verification processes, please visit these CTRP User Guide pages:
https://wiki.nci.nih.gov/display/CTRPdoc/Updating+Trial+Information
https://wiki.nci.nih.gov/display/CTRPdoc/How+to+Verify+Trial+Data
https://wiki.nci.nih.gov/display/CTRPdoc/How+to+Access+the+Trial+Data+Verification+Page
https://wiki.nci.nih.gov/display/CTRPdoc/Amending+Trials
July 2020
Flexible Accrual: Enhanced the CTRP accrual reporting capabilities to include more “Flexible Accrual” reporting options. Centers can now request to update the default accrual reporting type on a study registered in CTRP. Changing the default accrual reporting type requires a ticket to be submitted to the CTRO (NCICTRO@mail.nih.gov) for review and approval, before any change is granted.
The current default accrual reporting types are as follows:
Trial Type
Default Accrual Reporting Type
Complete Interventional
Subject
Complete Non-Interventional
Subject
Abbreviated/Imported Interventional
Summary
Abbreviated/Imported Non-Interventional
Summary
- CTRP is also introducing a new accrual reporting type, “Partial Subject.” Partial Subject accrual requires the reporting of Study Subject ID, Registration Date and Participating Site data only. The other accrual data fields are optional. The reporting of Partial Subject accrual also requires a ticket to be submitted to the CTRO (NCICTRO@mail.nih.gov) for review and approval, before any change is granted.
- Accrual Data Element changes:
- Study Subject Birth Date: For any/all ways to report accrual, only the month and year (MM/YYYY) are required to be collected.
- Study Subject Country: Required for all methods of reporting accrual (was previously optional)
- Disease:
- Study Subject: Required
- Partial Subject: Not required. However, for ICD-O-3 Disease Codes if Disease is reported, then Site must be reported.
- Site:
- Study Subject: Required
- Partial Subject: Not required. However, for ICD-O-3 Disease Codes if Site is reported, then Disease must be reported.
- Disease Codes: CTRP uses a hybrid model with the codes from IACR and the codes in NCI SEER that are not available in IACR.
For additional information on the CTRP Accrual process, including Flexible Accrual, Partial Subject accrual and other changes made within the Accrual application (e.g., ability to Delete All Trial Study Subjects,) please visit these CTRP User Guide pages:
https://wiki.nci.nih.gov/display/CTRPdoc/About+Accrual
https://wiki.nci.nih.gov/display/CTRPdoc/Searching+for+Trials+in+Accrual
If you would like to receive future CTRP announcements you may add yourself to the list here: https://list.nih.gov/cgi-bin/wa.exe?SUBED1=ctrp-users-l&A=1